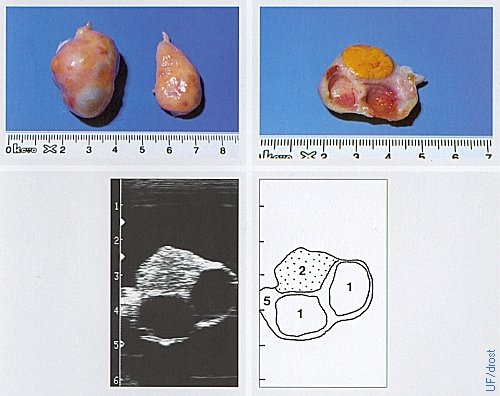
Ovaries on Day 0 of the Estrous Cycle.
Day 0 = estrus. The active left ovary shows a (functionally) regressed CL and 2 follicles. Legend: 1 = follicle, 2 = corpus luteum, 5 = ovarian stroma.
Pieterse MC (1999)
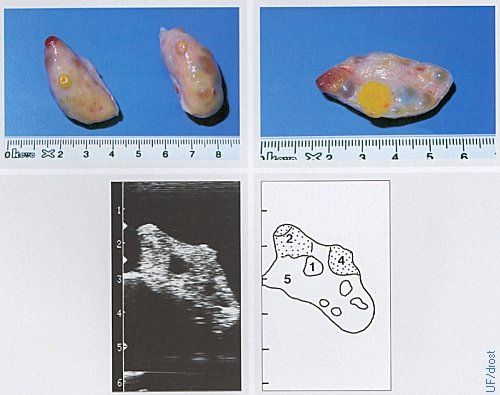
Ovaries on Day 2 of the Estrous Cycle.
The left ovary shows a corpus hemorrhagicum (red) which has a soft consistency, as well as a corpus albicans (yellow) which has a firm / dense consistency. Legend: 1 = follicle, 4 = regressed corpus luteum, 5 = stroma.
Pieterse MC (1999)

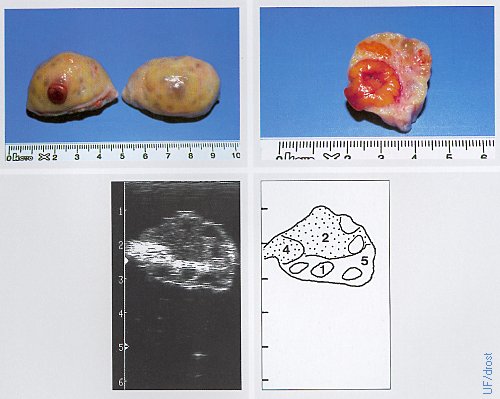
Ovaries on Day 3 of the Estrous Cycle.
The left ovary shows a corpus hemorrhagicum which has a soft consistency, as well as a corpus albicans which has a firm / dense consistency. Legend: 1 = follicle, 2 = corpus hemorrhagicum, 5 = stroma.
Pieterse MC (1999)
Ovaries on Day 4 of the Estrous Cycle.
The active left ovary shows a corpus hemorrhagicum and a dense corpus albicans. The right ovary shows an emerging dominant follicle. Legend: 1 = follicle, 2 = corpus hemorrhagicum, 4 = corpus albicans / atreticum, 5 = stroma.
Pieterse MC (1999)

Ovaries on Day 5 of the Estrous Cycle.
The left ovary shows a late corpus hemorrhagicum, the right ovary a dominant follicle. Legend: 1 = follicle, 2 = corpus hemorrhagicum, 5 = ovarian stroma, 8 = artifact.
Pieterse MC (1999)
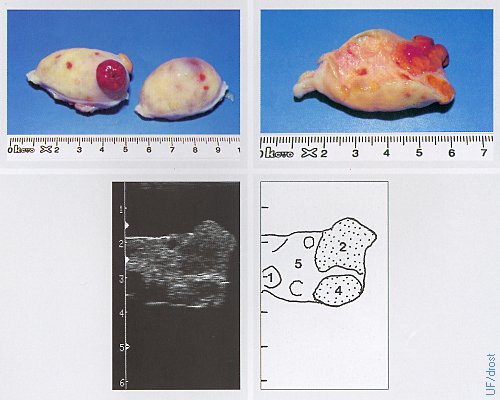
Ovaries on Day 6 of the Estrous Cycle.
The left ovary shows a late corpus luteum that is now becoming sensitive to the action of prostaglandin F2alpha. Legend: 1 = follicle, 2 = corpus luteum, 4 = corpus albicans, 5 = ovarian stroma.
Pieterse MC (1999)
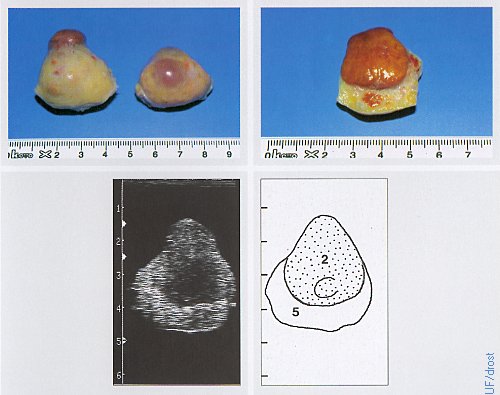
Ovaries on Day 7 of the Estrous Cycle.
The left ovary shows a fully developed corpus luteum, the right ovary a dominant follicle. Legend: 2 = corpus luteum, 5 = stroma.
Pieterse MC (1999)
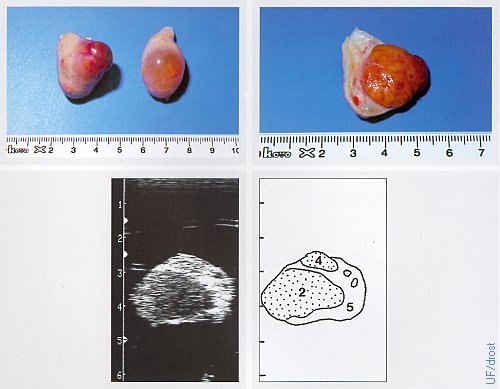
Ovaries on Day 9 of the Estrous Cycle.
The left ovary shows a corpus luteum, the right ovary a fully developed dominant follicle. Legend: 2 = corpus luteum, 4 = corpus albicans, 5 = stroma.
Pieterse MC (1999)
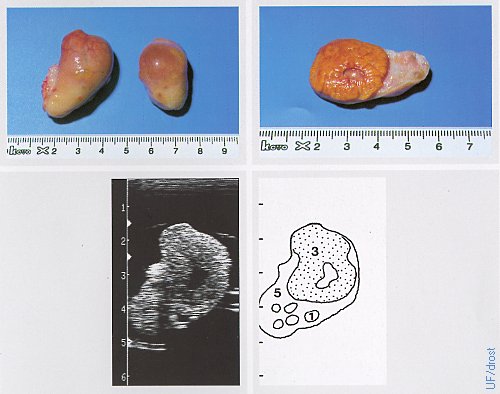
Ovaries on Day 14 of the Estrous Cycle.
The left ovary shows a fully developed corpus luteum, the right ovary a dominant follicle. Legend: 1 = follicle, 3 = corpus luteum with a small cavity (lacuna), 5 = ovarian stroma.
Pieterse MC (1999)
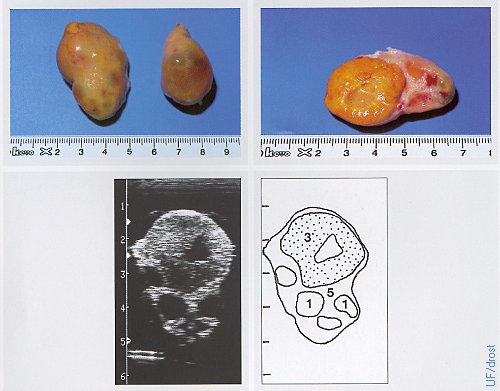
Ovaries on Day 15 of the Estrous Cycle.
The left ovary shows a fully developed corpus luteum with a smaller ovulation papilla than the one in the previous image, perhaps the result of the distension due to the fluid filled cavity which causes the CL to feel rounder. The right ovary shows a large dominant follicle. Legend: 1 = follicle, 3 = corpus luteum with a cavity (lacuna), 5 = stroma.
Pieterse MC (1999)
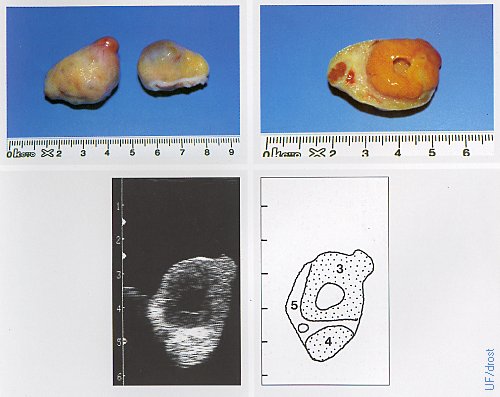
Ovaries on Day 16 of the Estrous Cycle.
The left ovary shows a fully developed corpus luteum, the right ovary shows a large dominant follicle. Legend: 1 = corpus luteum with a cavity (lacuna), 4 = corpus albicans, 5 = stroma.
Pieterse MC (1999)
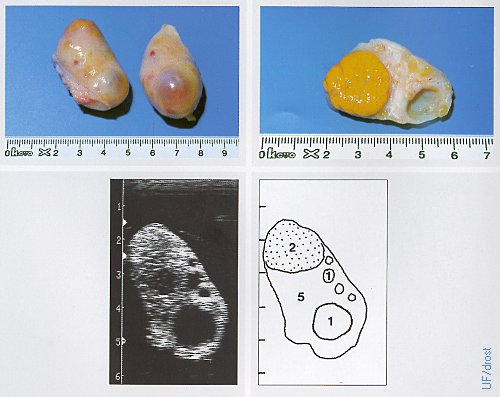
Ovaries on Day 17 of the Estrous Cycle.
The left ovary show a CL and a 12 mm follicle; the right ovary a 17 mm dominant follicle. Legend: 1 = follicle, 2 = corpus luteum, 5 = stroma.
Pieterse MC (1999)
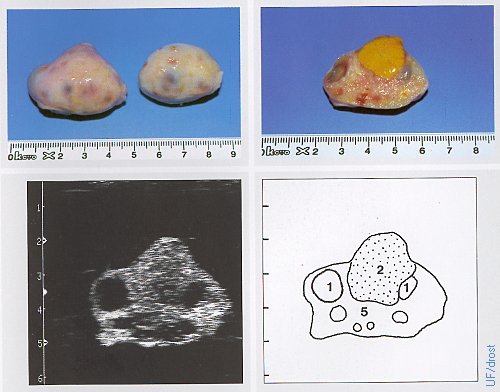
Ovaries on Day 20 of the Estrous Cycle.
The left ovary shows a regressing corpus luteum (which feels firmer than an active CL) and a dominant follicle. Legend: 1 = follicle, 2 = regressing CL, 5 = stroma.
Pieterse MC (1999)
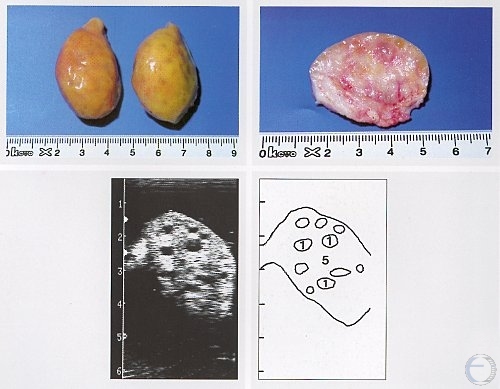
Inactive Ovaries
Inactive ovaries during anestrus (20 x 15 x 15 mm).
Pieterse MC (1999)
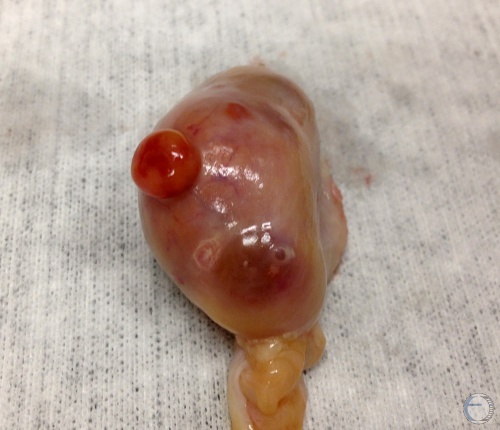
Corpus Hemorrhagicum.
Corpus hemorrhagicum on Day 3 to 4. A developing follicle is also present.
Denicol AC (2013)
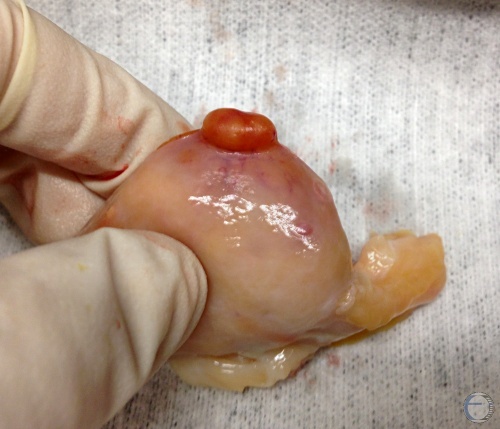
Corpus Hemorrhagicum.
Corpus hemorrhagicum on Day 3 to 4 with a small, soft ovulation papilla (crown).
Denicol AC (2013)
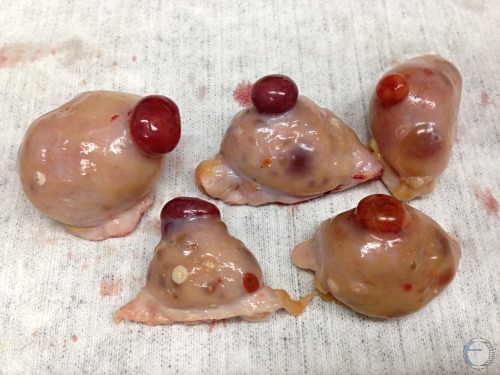
Corpora Hemorrhagica.
Corpora hemorrhagica on Day 3 to 4 with a small, soft ovulation papillae.
Denicol AC (2013)
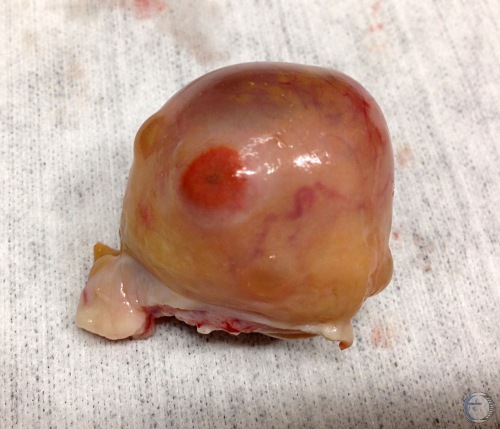
Cystic Follicle.
Cystic follicle and an old regressed corpus luteum.
Denicol AC (2013)
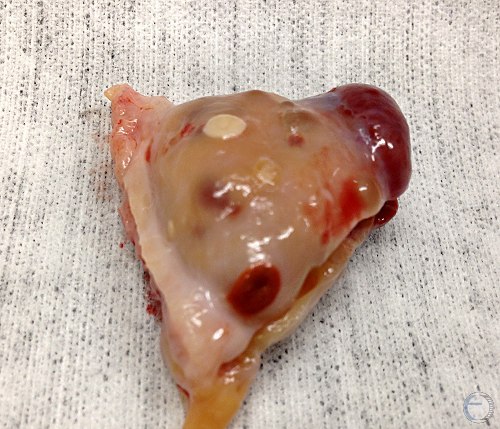
Multiple Structures.
Large diestrous corpus luteum, a firm regressed corpus luteum at the attached pole of the ovary, a small white scar of a corpus albicans, and several small follicles.
Denicol AC (2013)
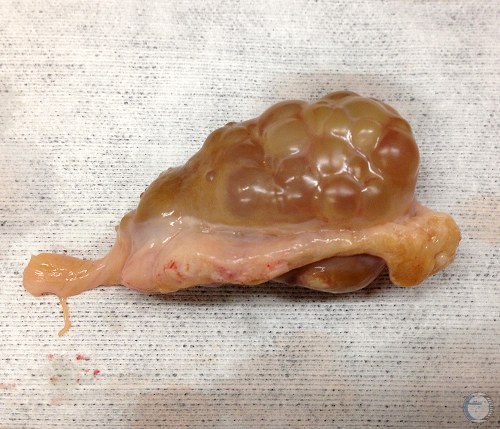
Superovulated Ovary.
Ovary in the early stage of superovulation for IVF oocyte collection or for embryo donors.
Denicol AC (2013)
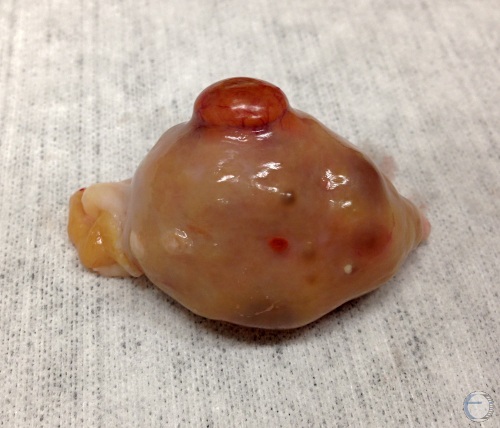
CL and Dominant Follice.
Diestrous corpus luteum and a dominant follicle. There are also some secondary follicles.
Denicol AC (2013)
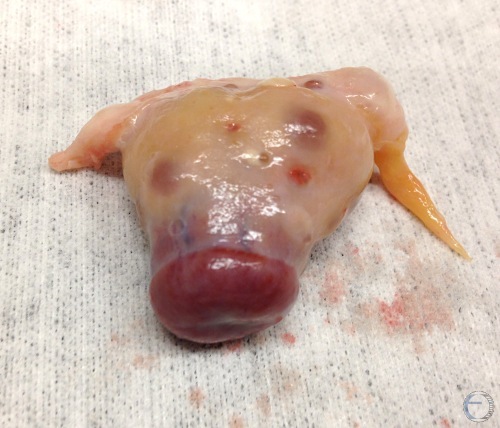
Diestrous Corpus Luteum.
Diestrous corpus luteum. Days 6 to 18 of the estrous cycle.
Denicol AC (2013)
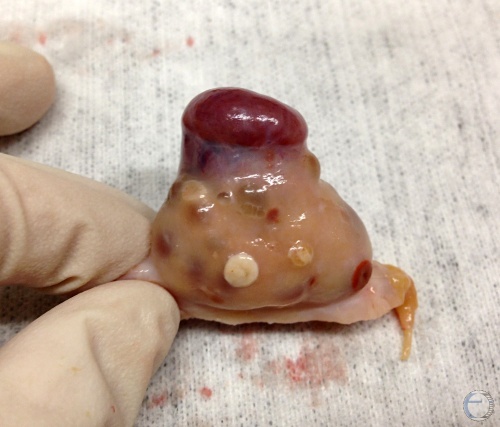
Corpus Luteum and Corpus Albicans.
Diestrous corpus luteum and a corpus albicans with a white ovulation papilla. There are also two older corpora albicantia.
Denicol AC (2013)
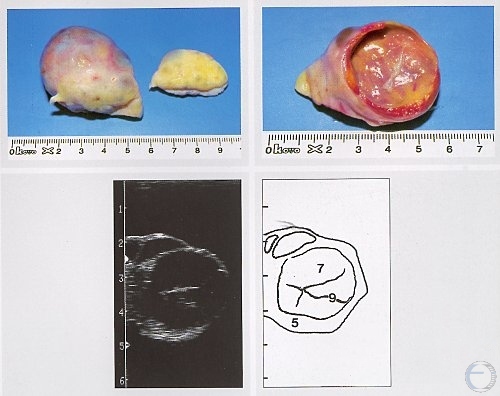
Luteal Cyst.
A thick-walled luteal (luteinized) cyst is shown. Cystic follicular degeneration is an anovulatory condition, hence there is no corpus luteum. Legend: 5 = stroma, 7 = luteal cyst, 9 = trabeculae.
Pieterse MC (1999)
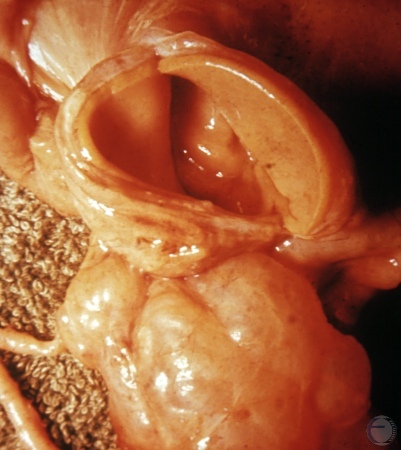
Large Luteal Cyst.
Unusually large luteinized follicular cyst. No evidence of an ovulation papilla hence it is not a cavitated corpus luteum.
Galvão K (2013)
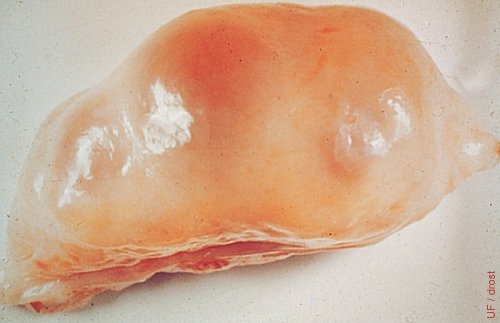
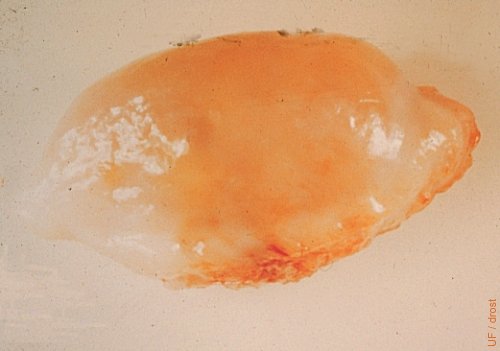
Ovary on Day 0 = Estrus.
A normal 15 to 20 mm preovulatory follicle.
Drost M (1974)
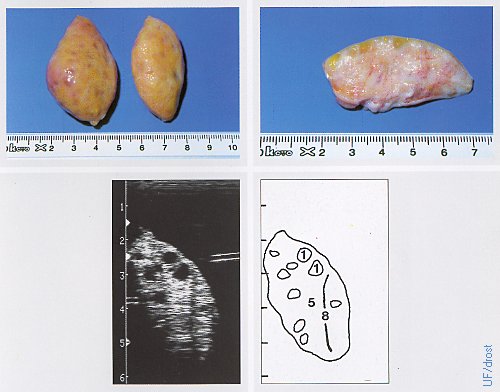
Ovaries on Day 7 Post Partum.
Inactive ovaries during the early postpartum period. Legend: 1 = follicle, 5 = stroma, 8 = artifact.
Pieterse MC (1999)
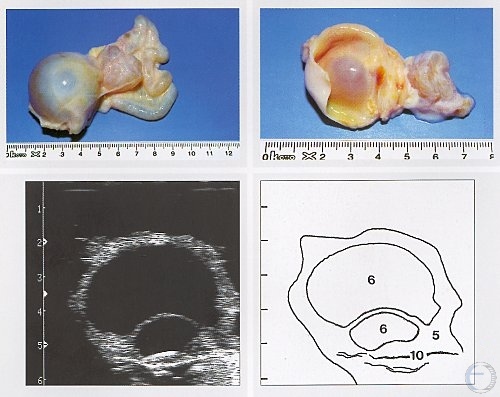
Follicular Cyst and Hydrosalpinx.
A large, thin-walled follicular cyst is shown accompanied by hydrosalpinx, a fluid-filled oviduct (uterine tube). Legend: 5 = follicle, 6 = follicular cyst, 10 = hydrosalpinx.
Pieterse MC (1999)
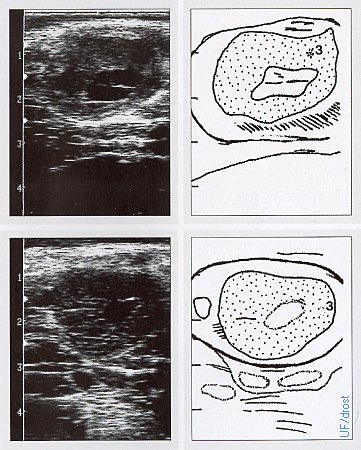
Ovaries on Days 7 and 10.
The upper panel shows a sonogram of a Day 7 ovary, the lower panel a Day 10 ovary (of the estrous cycle). Each corpus luteum has a cavity, also called a lacuna, which is a normal fluid filled cavity. These are frequently referred to as "cystic" CLs, perhaps an unfortunate term because they are normal functioning corpora lutea secreting the same amount of progesterone as a solid CL, hence no treatment is indicated. On palpation these hollow, or cavitated CL feel rounder and can be mistaken for a luteal cyst. They can be differentiated by determination of the progesterone concentration or by ultrasonography.
Pieterse MC (1999)
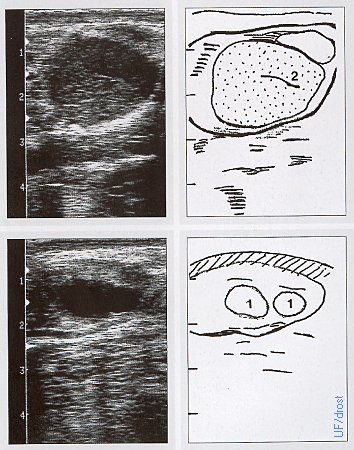
Normal Day 12 Ovarian Sonogram.
These are normal ovaries on Day 12 of the estrous cycle. Legend: 1 = follicle, 2 = corpus luteum.
Pieterse MC (1999)
Day 15 Ovarian Sonogram.
The left ovary in the upper sonogram shows 1 corpus luteum, the right ovary in the lower sonogram show shows 2 corpora lutea and a small follicle. Legend: 1 = follicle, 2 = corpus luteum, 3 = corpus luteum with a cavity, 9 = trabecula.
Pieterse MC (1999)

Ultrasound Unit with Transvaginal Probe.
Ultrasound unit with transvaginal probe with a sector scanner and a needle guide for folliculocentesis and recovery of oocytes in vivo.
Pieterse MC (1999)
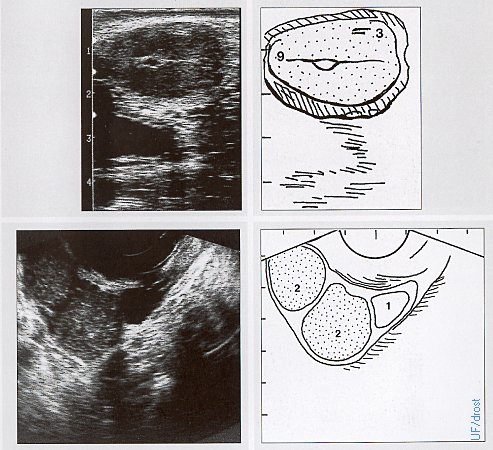
Anestrous Ovaries.
Normal symmetrical ovaries showing no activity (20 x 15 x15 mm).
Drost M (1970)

Kimberling-Rupp Instrument.
Kimberling-Rupp ovariectomy instrument. The instrument is introduced transvaginally into the peritoneal cavity, after which an ovary is placed in the oval window near the tip, by transsrectal manipulation. Next an internal shaft with a cutting edge is rotated to sever the ovary within the chamber. With a plunger the first ovary is pushed into the tip of the instrument, the window is re-opened and the second ovary is removed in the same manner. The instrument is primarily used for spaying feedlot heifers.
Drost M (1982)
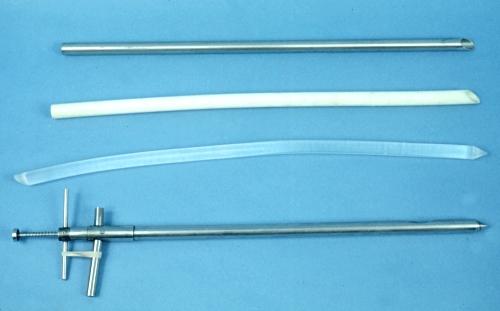
Colpotomy Instruments.
Homemade shafts / spears to penetrate the fornix in the cow to gain direct manual access to the abdominal cavity. The Kimberling-Rupp ovariectomy instrument, at the bottom, serves as a model with a length of 45 cm and a diameter of 2 cm. At the top is a stainless steel pipe with a beveled tip to resemble a giant needle. These can be made from a recycled IV stand or the leg of a small surgery table. Below it, is a piece of PVC pipe of the same dimensions. Next is a plexiglass rod again of the same dimensions. The PVC pipe and the plexiglass rod become bent / deformed upon autoclaving.
Drost M (1982)
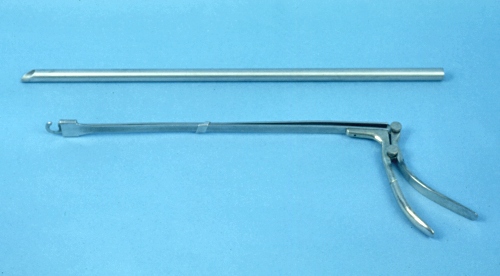
Colpotomy Instrument and Ecraseur.
A colpotomy instrument (length 64 cm) for penetrating the fornix is a cow or a mare. Below it, an ecraseur with a flat blade that slides through a slotted end.
Drost M (1982)
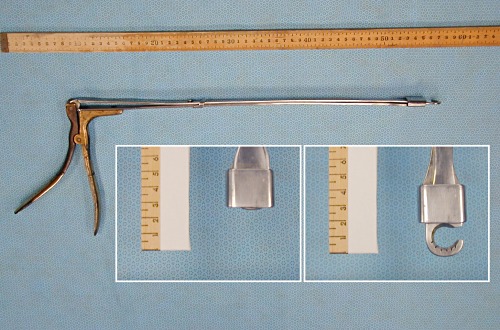
Ovarian Ecraseur.
This instrument can be used transvaginally (by colpotomy) to sever the ovarian pedicle. The curved tip, with a crush-cut blade, like an ecraseur, can be moved with the pistol grip handle as it slides within the square cuff of a second blade, as shown in the inset images. It is best to use this instrument on day 2 to 3 of the estrous cycle, when the ovarian blood supply is minimal.
Drost M (1982)
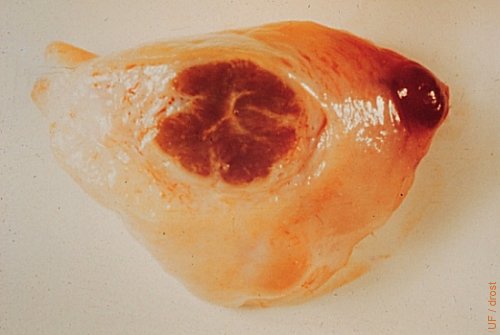
Corpus Hemorrhagiucm and Regressing CL.
A small hemorrhagic papilla is present on the right. A cross section of part of the ovary reveals a regressing corpus luteum in the process of becoming a corpus albicans. Day 2 of the estrous cycle.
Drost M (1970)
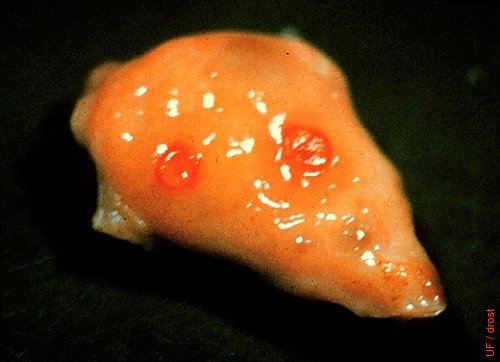
Twin Corpora Hemorrhagica.
Early corpora hemorrhagica. Day 2 of the estrous cycle.
Drost M (1970)
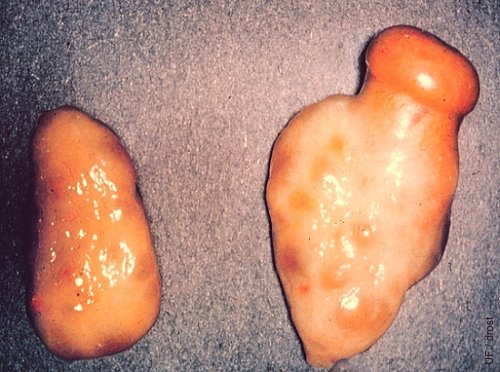
Midcycle CL.
A fully developed corpus luteum with a prominent ovulation papilla (crown) on the right ovary.
Drost M (1970)
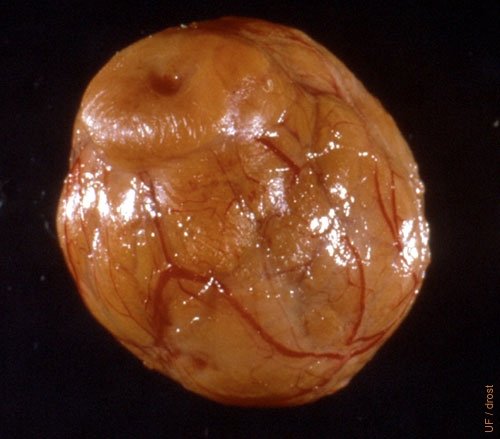
Mature Neat Corpus Luteum.
This is an entire diestrous corpus luteum dissected from a slaughter house specimen. Notice the rich vascularization.
Fields MJ (1999)
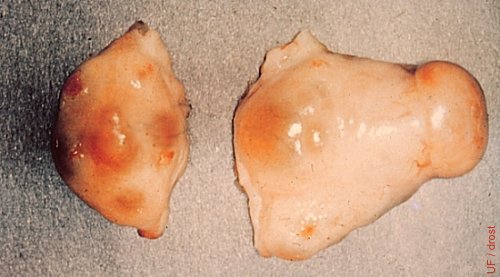
Mature CL and Dominant Follicle.
Active corpus luteum and a dominant follicle on the right ovary.
Drost M (1970)
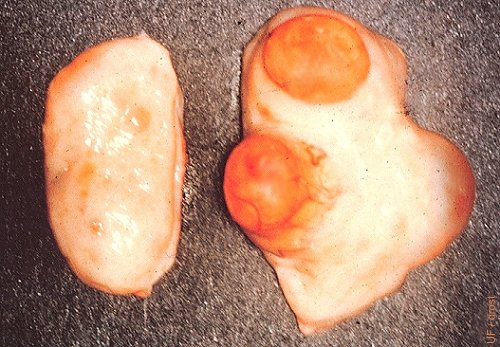
Twin CL and Dominant Follicle.
Fully developed twin corpora lutea and a dominant follicle.
Drost M (1970)
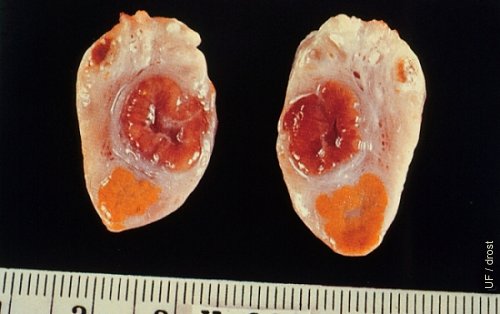
Regressed CL and Corpus Hemo.
Cross section of an ovary showing a firm, regressed, pale, yellow corpus luteum of the previous cycle (Day 24), and a soft corpus hemorrhagicum (Day 3) which does not have an obvious papilla.
Tanabe TY and Almquist GO (1966)
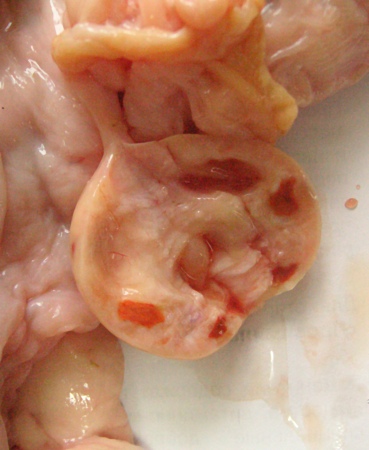
Corpora Albicantia.
This cross section of an inactive ovary shows several firm small corpora albicantia.
Plahotnik A (2007)
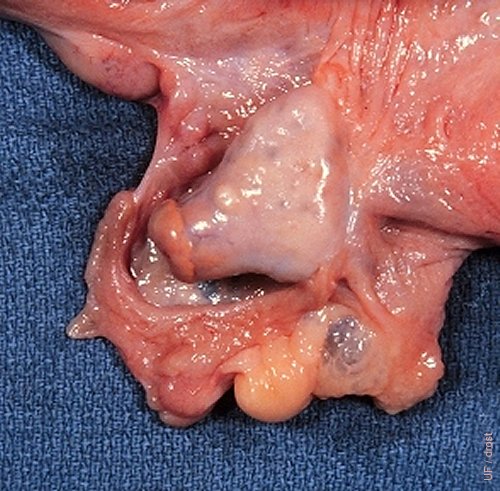
Ovary and Adnexa.
An active ovary with a fully developed CL is shown partially surrounded by the ovarian bursa.
Drost M (1970)
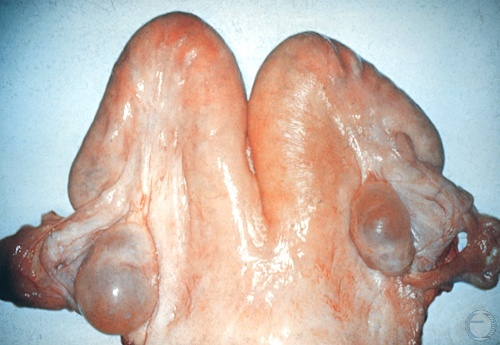
Follicular Cyst.
Follicular cysts are anovulatory structures. Notice the absence of a corpus luteum. Follicular cysts occur most commonly during the postpartum period (Days 15 to 45).
Drost M (1970)
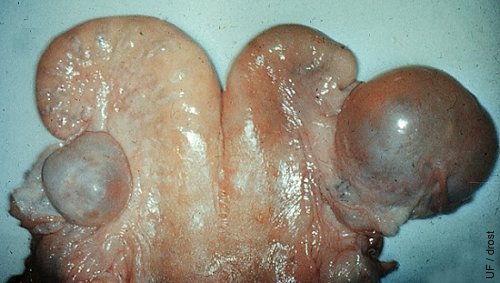
Cystic Follicular Degeneration.
Cystic follicular degeneration in the right ovary. This is a single, thin walled cyst that shows some early evidence of luteinization (near the tip of the horn). Two follicles are evident on the contralateral ovary.
Drost M (1971)
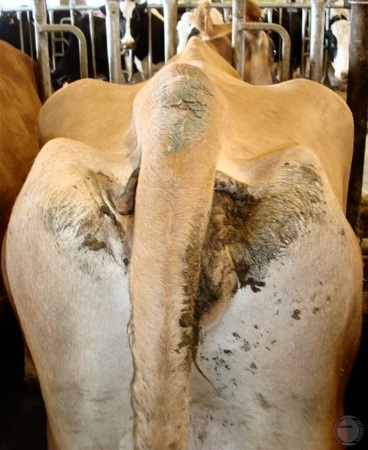
Sacrosciatic Relaxation.
Chronic cystic follicular degeneration can lead to relaxation of the sacro-ischiatic ligaments and the apparent elevation of the tailhead.
Villarroel A (2013)
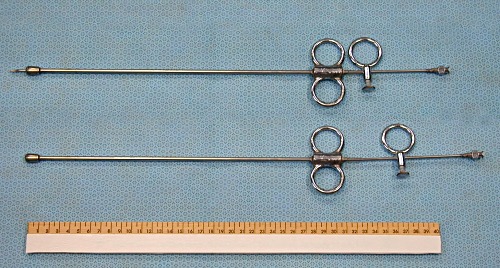
Stoll Cyst Aspirator.
Stoll cyst aspirator. A classical instrument used to aspirate the contents of follicular cysts per vaginam, while aligning the ovary per rectum. In the top instrument the needle has been advanced, in the bottom instrument the needle is still withdrawn within the protective, easily palpable, blunt tip.
Drost M (1971)
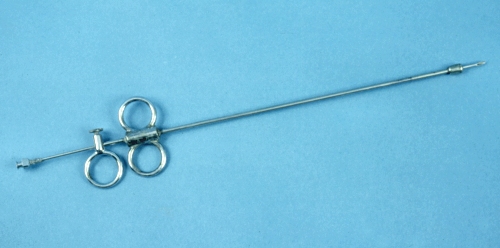
Stoll Cyst Aspirator - Assembled.
Stoll cyst aspirator. This classical instrument can be used to aspirate the contents of a follicular cyst.
Drost M (1971)
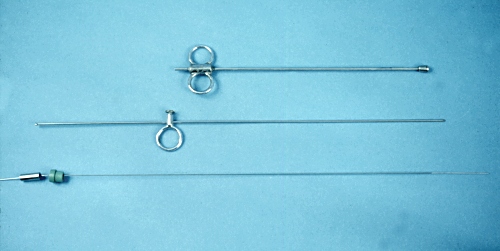
Stoll Cyst Aspirator - Disassembled.
Stoll cyst aspirator. The top portion is the outer shaft with an enlarged atraumatic knob at the end. The center portion is a large needle, which slides inside the shaft, and is used to penetrate the fornix. At the bottom is a small gauge aspirating needle which is advanced, via the large needle, into the cyst, after the cyst has been positioned, transrectally, against the blunt end of the instrument, which is still in the fornix.
Drost M (1971)

Tip of Cyst Aspirator.
Stoll cyst aspirator. Tip of the large needle, which slides inside the shaft, is used to penetrate the anterior wall of the fornix.
Drost M (1971)

Aspiration Needle.
The aspiration needle emerges from the large needle which is used to penetrate the anterior wall of the fornix. The enlarged knob of the instrument remains in the vagina.
Drost M (1971)
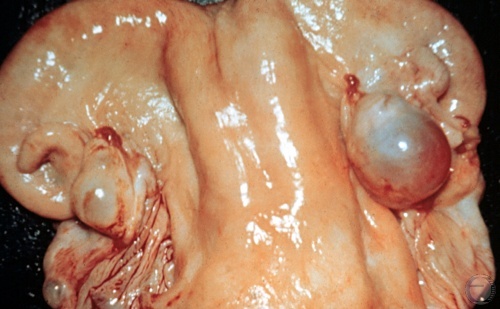
Follicular Cyst.
A single, thin-walled follicular cyst is shown. Many of these cysts readily collapse on palpation per rectum. Cysts should not be ruptured deliberately, lest hemorrhage ensues and adhesions form. Most cysts may be treated successfully with GnRH.
Drost M (1969)
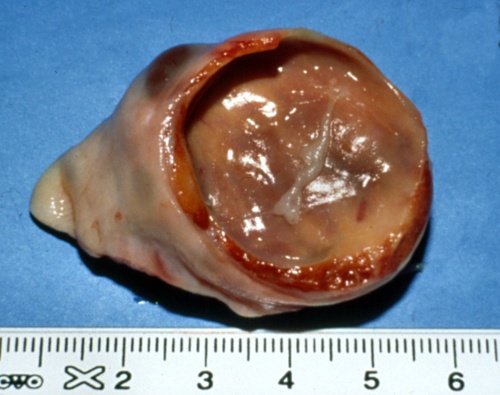
Thick Walled Cyst.
A thick-walled luteal (luteinizing) cyst is shown. Cystic follicular degeneration is an anovulatory condition, hence there is no corpus luteum.
Pieterse MC (1999)
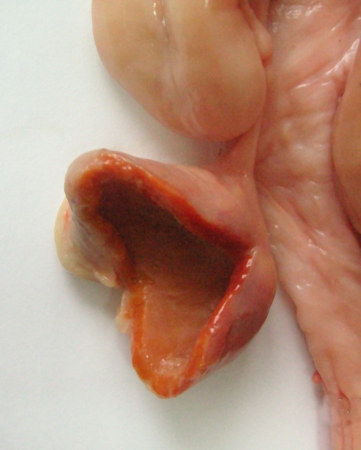
Luteinizing Follicular Cyst.
This is the transition stage from a follicular cyst to a luteal cyst. These structures are a diagnostic challenge on palpation of the ovary. They can be more clearly defined with ultrasonography. There is no ovulation papilla.
Plahotnik A (2007)

Cystic Corpus Luteum.
The right ovary shows a bulging, round corpus luteum that may be mistaken for a luteal cyst on palpation because of its shape. However, there is an ovulation papilla. The term cystic CL is perhaps unfortunate because these cavitated CL produce the same concentration of progesterone as a solid CL, hence they function normally.
Drost M (1970)

Nymphomania.
Elevation of the tailhead in this cow is the result of chronic cystic follicular degeneration which has led to chronic relaxation of the pelvic ligaments. These cows show frequent behavioral estrus. In the vernacular they are called buller cows. They can be of help in a heat detection program, however their behavior is not consistent. The elevated tailhead is sometimes referred to as the sterility hump.
Drost M (1970)

Nymphomaniac Holstein Cow.
Elevation of the tailhead in this cow is the result of chronic cystic follicular degeneration which has led to chronic relaxation of the pelvic ligaments. These cows show frequent behavioral estrus. In the vernacular they are called buller cows. They can be of help in a heat detection program, however their behavior is not consistent. The elevated tailhead is sometimes referred to as the sterility hump.
Utrecht (1976)
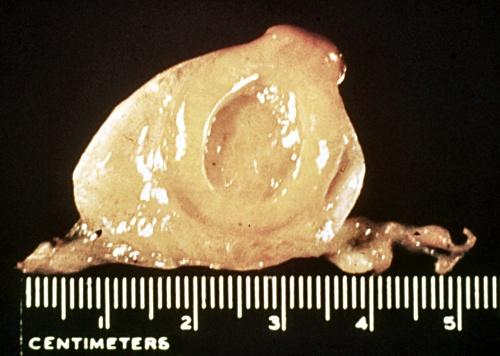
Cystic Corpus Luteum.
Cavitated or hollow corpora lutea are normally functioning corpora lutea with a central space, a lacuna. They are commonly referred to as cystic corpora lutea which is technically correct because they do contain a cavity, an encystment. The term leads to confusion, especially on the part of the layman who expects them to require treatment. Hollow corpora lutea produce as much progesterone as normal solid CL, hence do not require treatment.
Roberts SJ (1973)
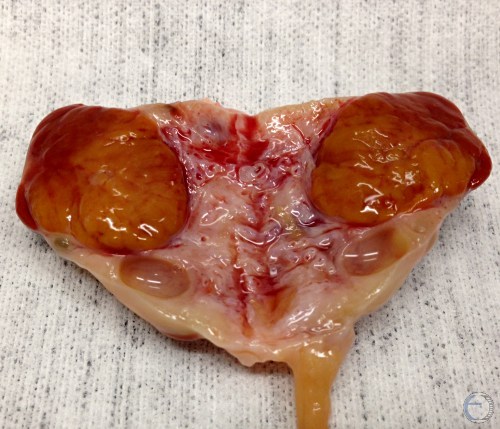
Cross Section of a CL.
Cross-section of a normal mature corpus luteum.
Denicol AC (2013)
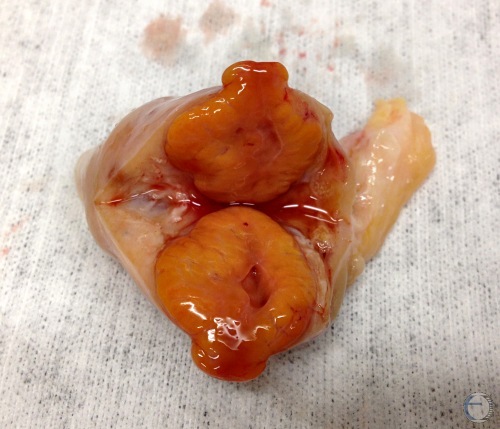
"Hollow" Corpus Luteum.
Cross-section of a mature corpus luteum with small cavitation. The hollow space can be confusing on ultrasound examination and / or palpation. Functionally they are normal.
Denicol AC (2013)
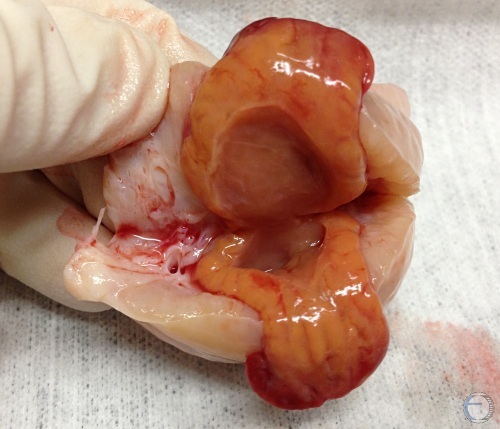
"Hollow" Corpus Luteum.
Cross-section of a mature corpus luteum with sizable cavitation. The hollow space can be confusing on ultrasound examination and / or palpation. Functionally they are normal. The total amount of luteal tissue is produced and the normal amount of progesterone.
Denicol AC (2013)
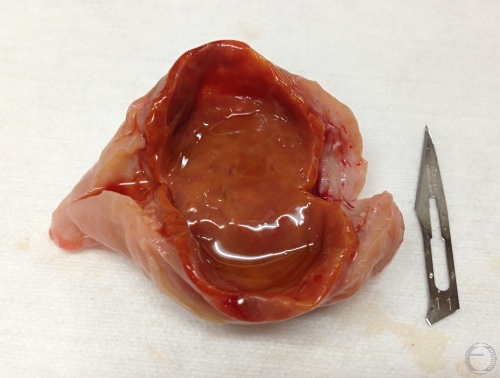
Cavitated Corpus Luteum.
Cavitated or hollow corpora lutea can produce as much progesterone as normal solid CL. They are a diagnostic challenge especially by transrectal palpation. Ultrasonography helps. This CL has an ovulation papilla.
Denicol AC (2013)
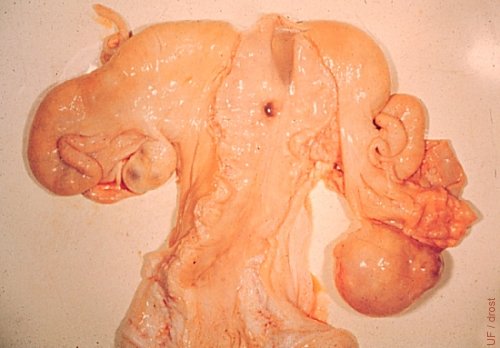
"Cystic" CL in a Pregnant Cow.
This gravid tract confirms that a "cystic" corpus luteum is functionally normal. Notice the early conceptus in the (opened) right horn.
Drost M (1971)
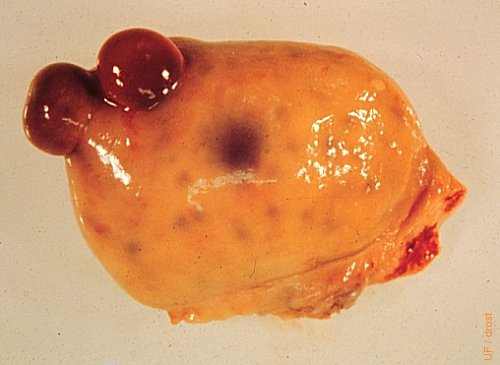
Germinal Inclusion Cysts.
Germinal inclusion cysts are rare. They are endocrinologically inactive, hence not related to any stage or phase of the reproductive cycle. On transrectal palpation they may be mistaken for corpora hemorrhagica or lutea. Their close proximity to one another adds to the confusion.
Roberts SJ (1973)
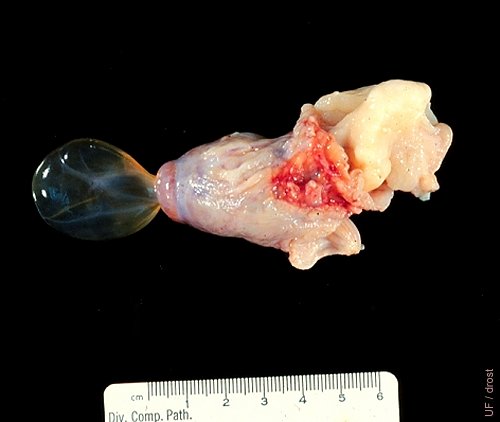
Peritoneal Inclusion Cyst.
Slaughterhouse specimen. Peritoneal inclusion cyst? A rare specimen. Confusing to palpate or visualize by ultrasound. Likely to be insignificant functionally.
Drost M (1970)
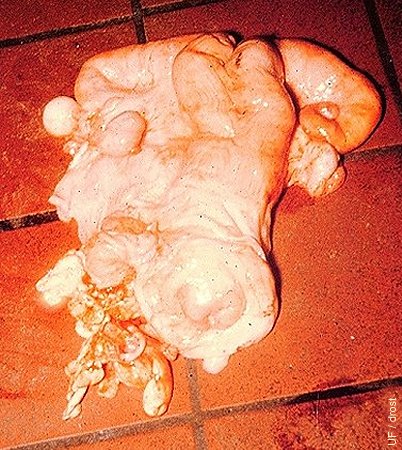
Parovarian Cyst.
Parovarian cyst associated with the left ovary. These cyst are frequently remnants of the (male) mesonephric duct system. They are confusing to the palpater. Unless they are obstructive, they do not cause infertility.
Roberts SJ (1973)
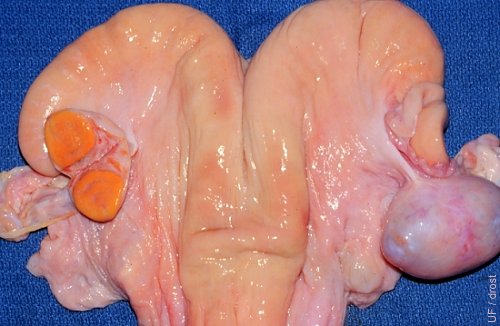
Ovarian Cyst.
The right ovary shows a cyst of undetermined origin but likely the result of trauma. Such a cyst is difficult to differentiate from a follicular or luteal cyst by palpation or ultrasonography only. However, the cyst is endocrinologically inactive which is confirmed by the presence of a normal midcycle corpus luteum on the contralateral ovary.
Roberts SJ (1973)
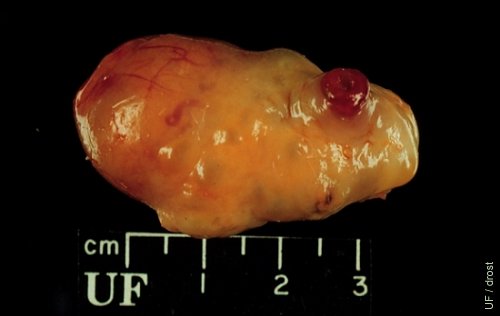
Accessory Corpus Luteum.
This ovary shows a fully developed corpus luteum (left one-third of the ovary) and the papilla of a (3-day old) corpus hemorrhagicum. This is not a natural or spontaneous occurrence. Cows do not ovulate in the face of elevated progesterone concentrations. However, dominant follicles, in the presence of a corpus luteum, can be made to ovulate by the administration of exogenous GnRH or LH, and create an accessory CL.
Drost M (1970)
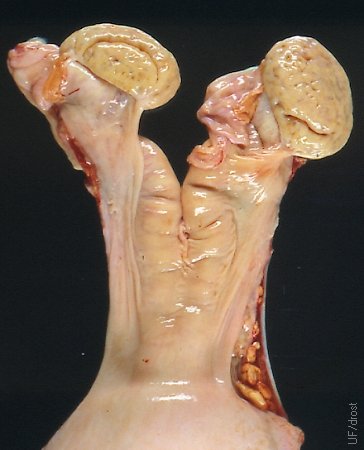
Pancake Ovaries.
Bos indicus ovaries frequently have a flattened appearance, resembling oatmeal cookies or small pancakes, hence the colloquial name. They are normal for this species of cattle. Corpora lutea and follicles tend to remain largely below the surface making them difficult to palpate.
Drost M (1979)
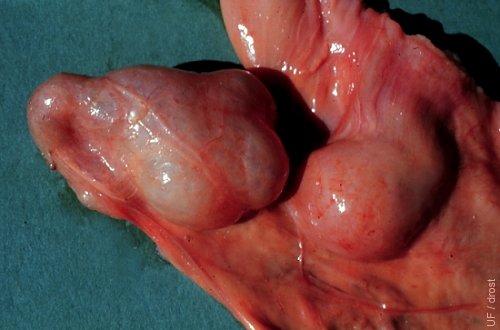
Ovarian Adhesions.
Periovarian tissues are smoothly adhered to the ovary preventing ovulation. These ovaries are difficult to palpate and delineate. If bilateral, the animal is sterile.
Drost M (1970)
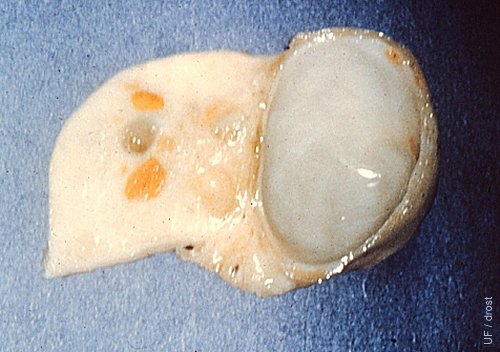
Paroophoron.
Incidental finding on a slaughterhouse specimen. Paroophoron, a remnant of the mesonephric duct system, which is functionally insignificant, hormonally inactive, and diagnostically challenging.
Roberts SJ (1973)
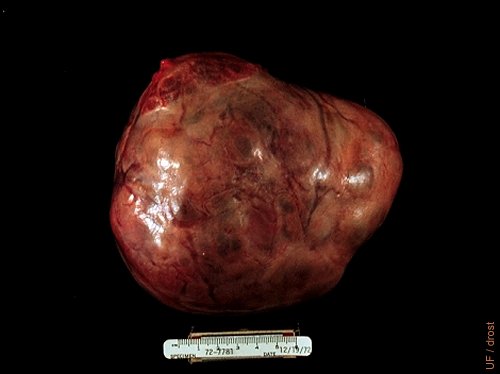
Cystic Granulosa Cell Tumor.
Granulosa cell tumor surgically removed from a nulliparous Holstein heifer. Approximate diameter: 14 cm.
Drost M (1973)

Sagital Section of a GCT.
Sagital section of a cystic granulosa cell tumor. The multi-locular appearance can be visualized ultrasonographically. The surface on palpation per rectum has a hobnail feel to it.
Drost M (1973)
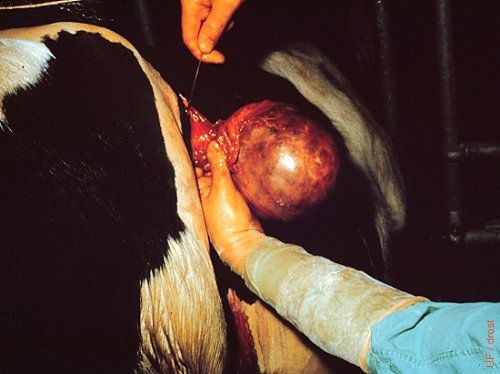
Surgical Removal of a GCT.
Surgical removal of a granulosa cell tumor via the ipsilateral flank from an 18-month old nulliparous Holstein heifer. Approximately 14 cm in diameter. Careful ligation of the ovarian artery is required. These tumors should never be severed with an ecraseur alone.
Drost M (1973)

Holstein Heifer with a GCT.
The tailhead of this heifer is becoming elevated due to presence of a chronic granulosa cell tumor. The contralateral ovary was inactive. The tumor was surgically removed (see preceding three images).
Drost M (1973)
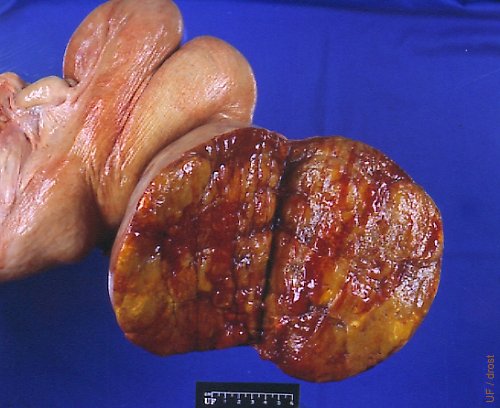
Solid Granulosa Cell Tumor.
Bovine granulosa cell tumors may be polycystic or solid (as shown here).
Drost M (1974)
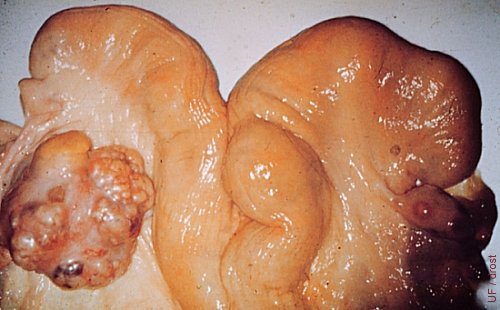
Small Granulosa Cell Tumor.
This small granulosa cell tumor does not (yet) interfere with the cyclicity of the animal. Note the corpus luteum on the contralateral ovary. Affected ovaries present a diagnostic problem. They must be monitored over time.
Roberts SJ (1973)
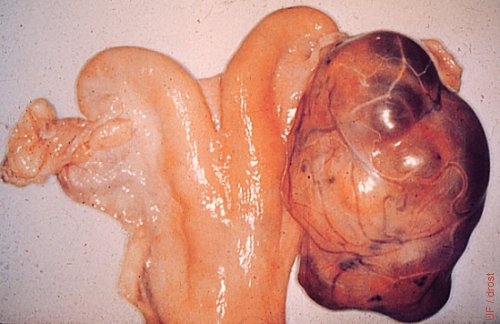
Large Granulosa Cell Tumor.
The heifer acted like a bull and showed aggressive behavior.
Roberts SJ (1973)
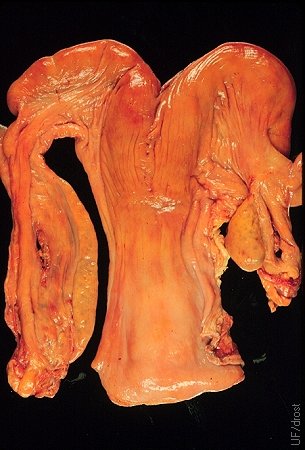
Ovarian Hypoplasia.
Complete congenital hypoplasia of the left ovary. The right ovary is normal judging by the fact that the cow has been pregnant in the right horn. The elongated ovary is also referred to as a streak gonad.
Drost M (1971)
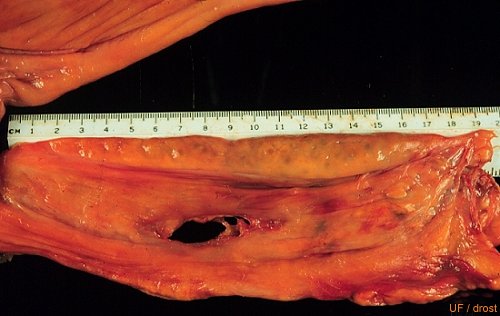
Streak Gonad.
Streak gonad: 18 cm long. Congenital hypoplasia of the ovary. See previous image.
Drost M (1971)
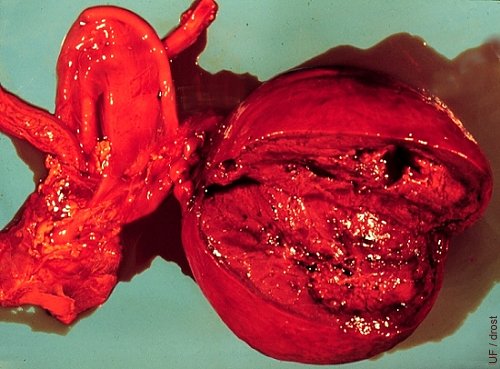
Hamartoma.
Congenital anomaly of the ovary of a fetus showing a rare vascular hamartoma.
Roberts SJ (1973)
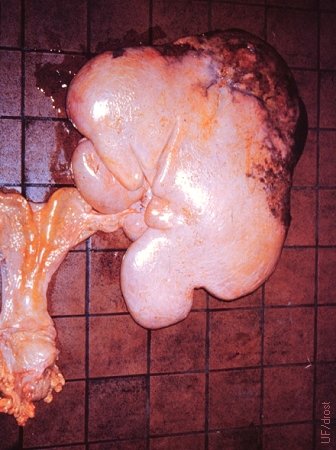
Fibromatous Hamartoma.
33 kg tumor, 60 x 50 x 20 cm. A vascular fibromatous hamartoma. There were chronic cysts on the contralateral ovary. The uterus is atrophied. [The size of the square tiles is 15 cm].
Roberts SJ (1973)
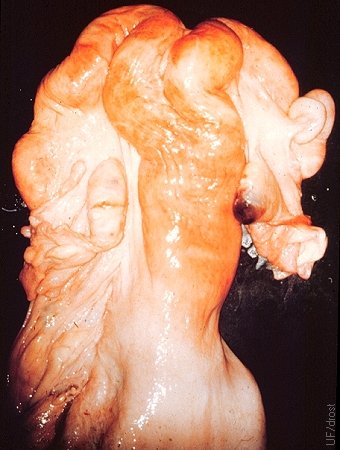
Stellate Scar.
The left ovary shows a stellate scar in evidence of the manual enucleation (expression) of a corpus luteum. The right ovary shows a normal corpus hemorrhagicum. The practice of enucleation was relatively common prior to the advent of prostaglandin F2alpha, to initiate a new cycle. Hemorrhage and the formation of adhesions were undesirable side effects. Occasionally a cow suffered a fatal hemorrhage.
Roberts SJ (1973)
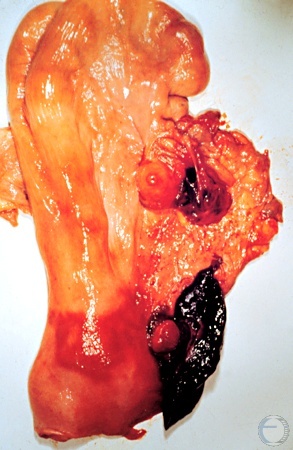
Enucleation of the Corpus Luteum.
Enucleation of the corpus luteum by digital expression per rectum was practiced prior to the availability of prostaglandin F2alpha. It was followed by estrus in 3 days. Hemorrhage and the formation of adhesions were undesirable side effects. Occasionally a cow suffered a fatal hemorrhage. The enucleated CL is shown in this image on the left side of the large blood clot in the broad ligament at the level of the cervix. There is also a small blood clot in the ovarian bursa. In the meantime a new CL has formed in the right ovary where it can be identified by its ovulation papilla.
Roberts SJ (1973)
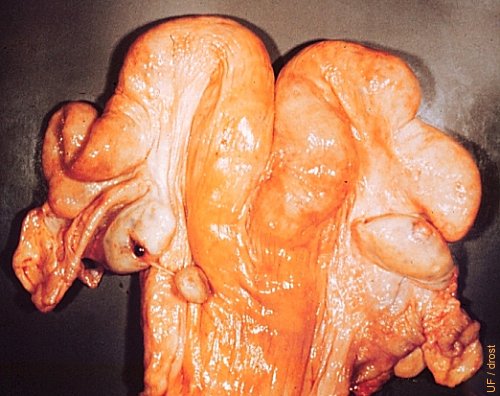
Enucleated Corpus Luteum.
The manually enucleated corpus luteum remained attached to the left ovary by a strand of connective tissue. A normal (subsequent) diestrous corpus luteum is present on the right ovary.
Roberts SJ (1973)
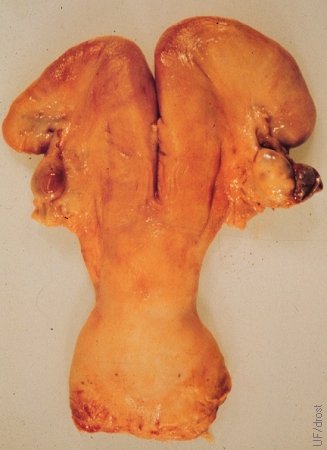
Necrotic Enucleated CL.
A necrotic enucleated corpus luteum, retained in the right ovarian bursa, led to the formation of adhesions.
Roberts SJ (1973)
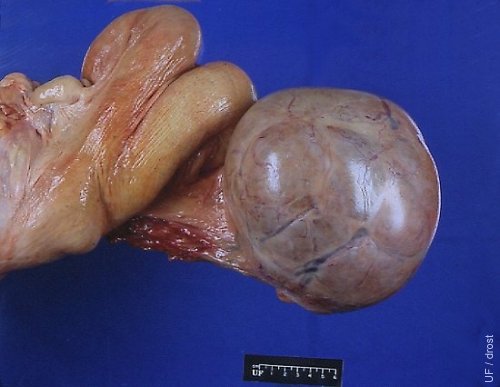
Smooth Granulosa Cell Tumor.
On palpation per rectum this granulosa cell tumor felt smooth and firm. The presence of a corpus luteum on the contralateral ovary suggests that the tumor is endocrinologically inactive.
Buergelt CD (1999)
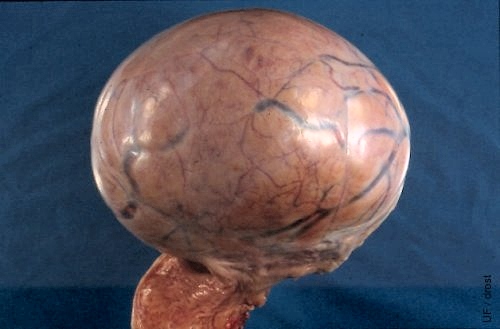
Smooth Granulosa Cell Tumor.
Some granulosa cell tumors have a lobular appearance and feel. In contrast, this is a solid tumor which was histologically classified as a granulosa cell tumor. The prominent blood vessels serve as a reminder that surgical removal requires careful ligation of the blood supply. It is not safe to merely crush the pedicle with an ecraseur or emasculator.
Buergelt CD (1999)
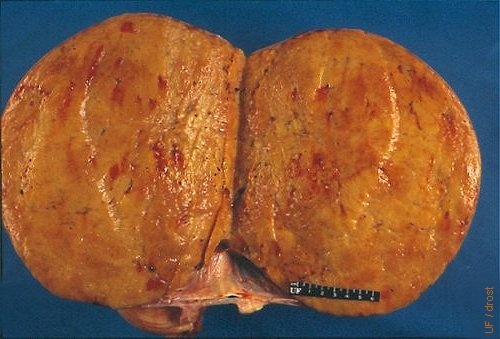
Solid Granulosa Cell Tumor.
Cross section of a solid, smooth feeling granulosa cell tumor. Histologically it was classified as a granulosa cell tumor.
Buergelt CD (1999)
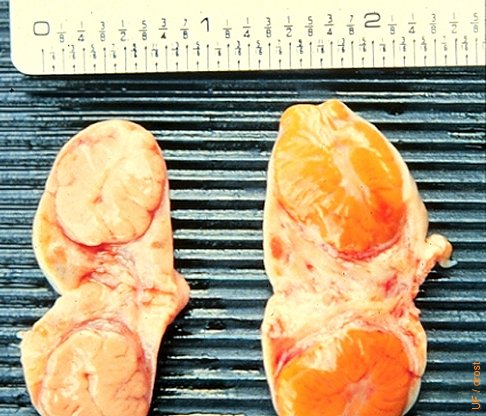
Embedded Corpus Luteum.
Comparison of an embedded corpus luteum in the left ovary with a normal CL in the right specimen. Normally the ovulation papilla (crown) can be palpated. When there is no distinct crown the CL may be difficult to identify. The presence of a mature CL can be confirmed with ultrasonography and / or progesterone determination in the milk or plasma.
Drost M (1971)
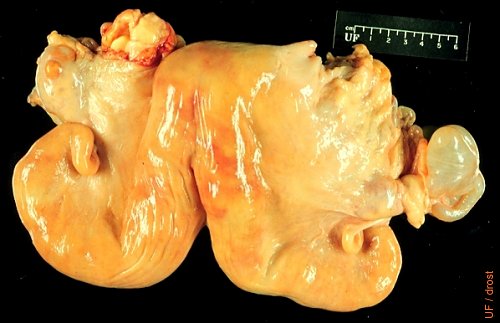
Hydrosalpinx.
The left oviduct is grossly distended with fluid. This structure presents a challenge in differential diagnosis with ovarian cysts. The contralateral ovary displays a corpus luteum which rules out an endocrine problem. The cow is subfertile and can only conceive in the right horn.
Drost M (1971)
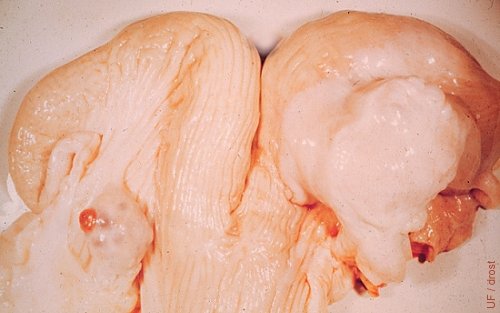
Ovarian Abscess.
The right ovary is abscessed. The abscess is confined to the ovary and the immediate periovarian area. While unknown in this case, the abscess may have formed as an extension of a uterine infection via the oviduct to a fresh corpus hemorrhagicum. The contralateral ovary shows a corpus hemorrhagicum.
Drost M (1971)
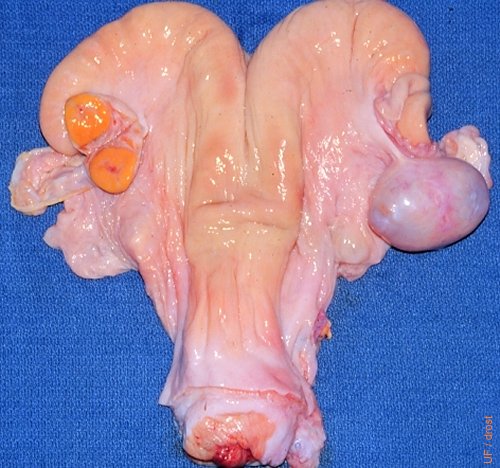
Inactive Cyst.
The right ovary contains a large fluid filled structure ("cyst") which is endocrinologically inactive, to wit, the presence of an active corpus luteum on the left ovary. The fluid filled structure presents a diagnostic challenge to the palpater and the ultrasonographer. There is evidence of adhesions.
Drost M (1971)
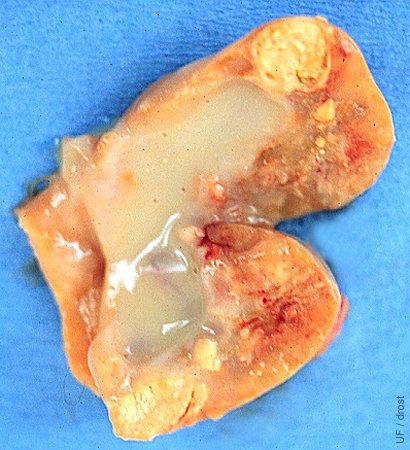
Intraovarian Abscess.
A small intraovarian abscess is present in this ovary. In the live cow this presents a diagnostic challenge to differentiate the small firm abscess from a corpus luteum. Of course, the abscess is endocrinologically inactive, hence unlikely to affect the cyclic activity. Rupture will predispose to adhesions.
Drost M (1971)
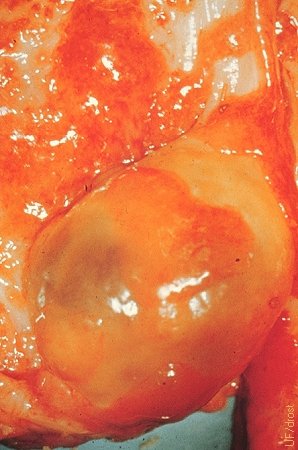
Ovarian Tuberculosis.
The granulomatous reaction shown by this ovary is due to tuberculosis.
Roberts SJ (1973)
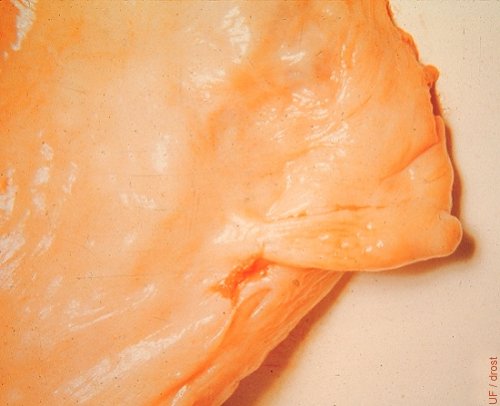
Partial Ovarian Hypoplasia.
Partial congenital hypoplasia of the medial pole of the ovary. This occurs infrequently.
Roberts SJ (1973)
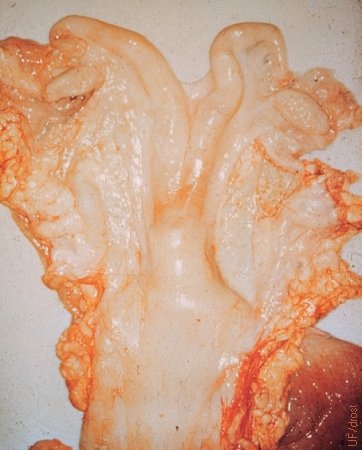
Acute Vitamin A Deficiency.
The ovaries of this heifer were inactive due to acute vitamin A deficiency as a result of (experimental) poisoning with chlorinated naphthalenes, formerly used in lubricants.
Roberts SJ (1973)

Ovariectomy.
Pistol grip ecraseur, with a flat crush-cut blade, for ovariectomy by laparotomy or colpotomy in cattle. It is best to use this instrument on day 2 to 3 of the estrous cycle, when the ovarian blood supply is minimal.
Drost M (2009)