The Visual Guide to
Bovine Reproduction
Reproductive Technology: Embryo Transfer

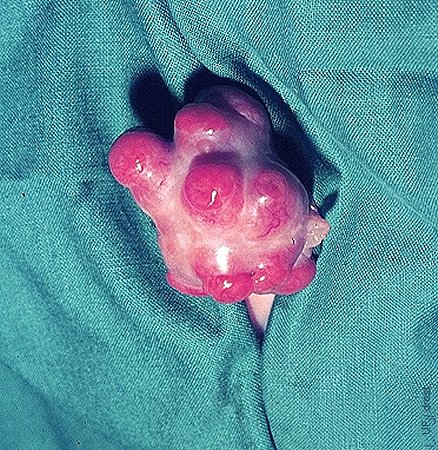
Donor with Litter of Calves.
Holstein donor cow with six of her calves carried by beef cattle recipients as surrogate dams.
Seidel GE (1984)
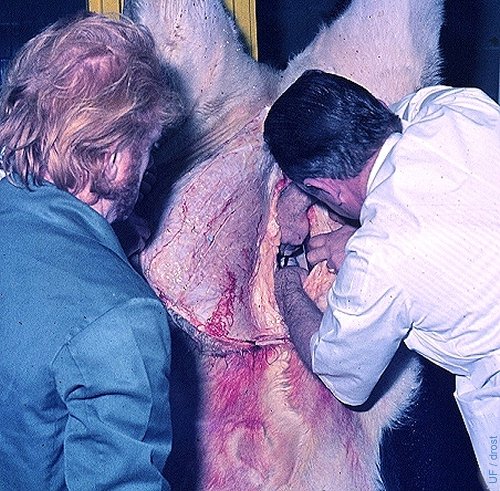
Recovery at Slaughter.
Recovery of the uterus and ovaries from a slaughtered animal prior to embryo recovery.
Brand A (1975)
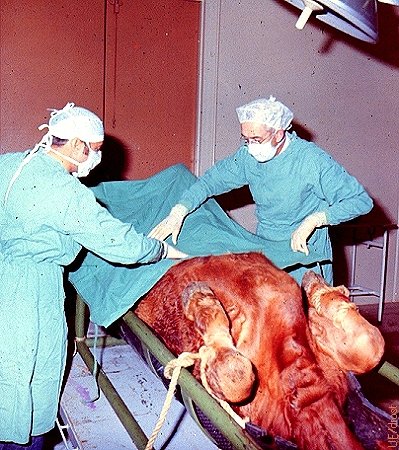
Draping the Abdomen.
Draping the abdomen in preparation for surgical recovery of embryos with the donor under general anesthesia. Surgery is performed via the midline.
Drost M (1973)
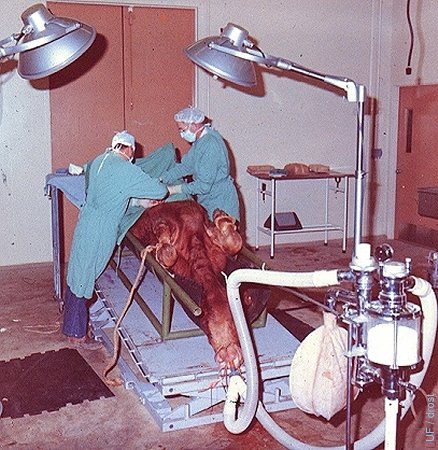
Surgical Recovery.
Midline incision for exposure of the uterine horns for recovery of embryo from a superovulated donor under general inhalation anesthesia.
Drost M (1973)
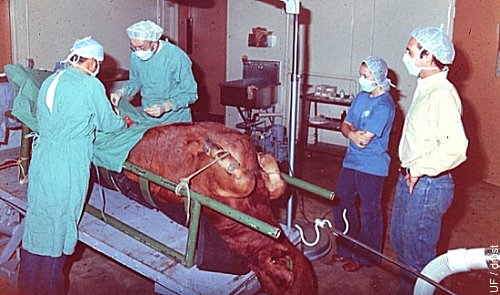
Donor Under General Anesthesia.
Donor in dorsal recumbency under general inhalation anesthesia. The surgery table is tilted to facilitate access to the reproductive tract via a midline incision just anterior to the udder.
Drost M (1973)
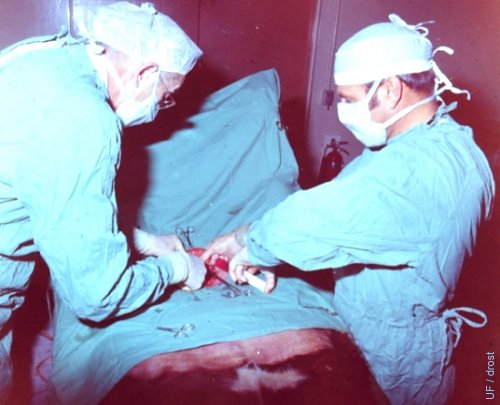
Donor in Dorsal Recumbency.
Anesthetized donor in dorsal recumbency. Flushing in progress.
Drost M (1973)
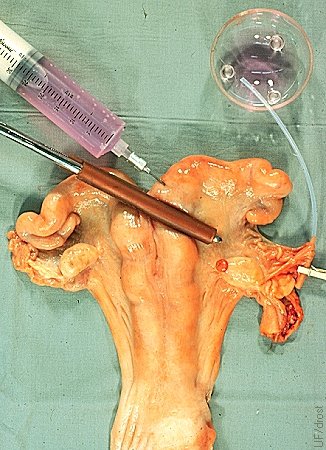
Oviductal Catheter Placement.
The base of the uterine horn is occluded with a rubbershod Doyen intestinal forceps. The injection site at the base of the horn is shown. The oviductal catheter is held in place with a miniature clothes pin. Oviductal flushes are done on Day 3.
Drost M (1974)
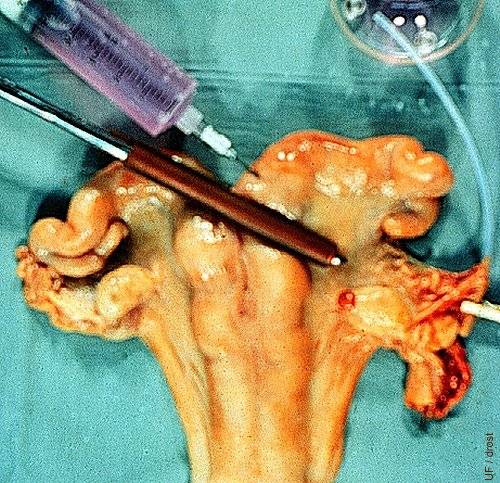
Surgical Flush.
In this mock-up of surgical recovery of bovine embryos on Day 4, 1) the base of the uterine horn is clamped with a rubber-shod Doyen (intestinal) forceps; 2) the oviduct is cannulated with a fine 2mm OD sylastic catheter; 3) flushing medium is injected at the base of the horn to flush the embryo from the oviduct into a sterile Cambridge searching dish. Notice the corpus hemorrhagicum.
Drost M (1974)
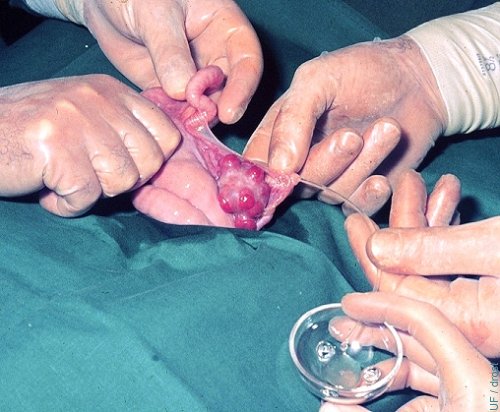
Oviductal Flush - Superovulation.
A small diameter Teflon catheter has been inserted into the oviduct and the ipsilateral uterine horn is flushed from its bifurcation, on Day 3 of the cycle of a superovulated donor.
Drost M (1974)
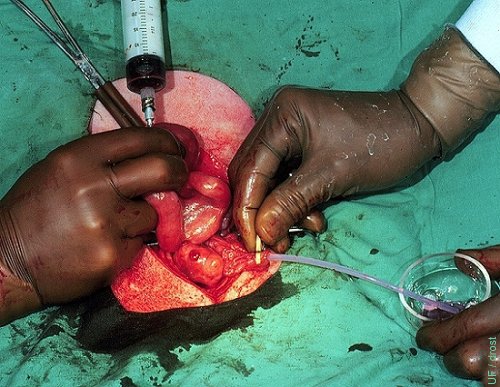
Oviductal Flush - Natural Cycle.
A small diameter Teflon catheter has been inserted into the oviduct and the ipsilateral uterine horn is flushed from its bifurcation on Day 3 of a natural cycle. Note corpus hemorrhagicum.
Drost M (1974)
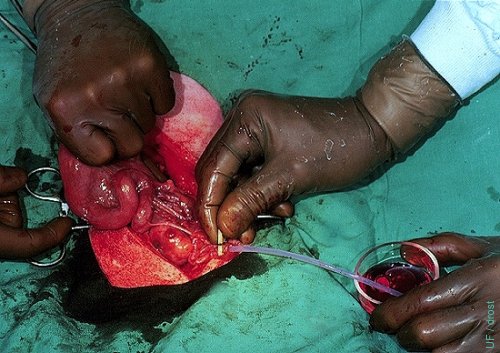
Oviductal Flush on Day 3.
The flushing medium has been injected and is massaged toward the tip of the horn.
Drost M (1974)
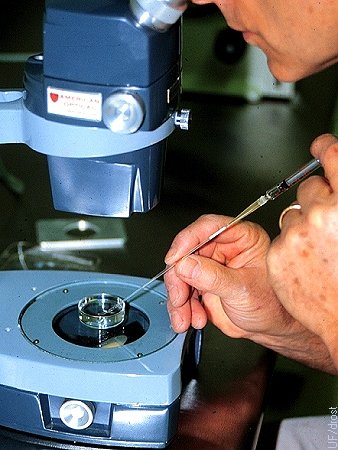
Loading the Straw.
Loading the embryo from the holding dish into a 0.25 cc straw attached to a tuberculin syringe.
Drost M (1974)

Heifer Recipients.
A group of 15 month old cycling Holstein heifers in a holding pen in South Florida.
Drost M (1984)

Dairy Cow Recipients.
Commercial dairy cows in the sprinkler pen waiting to be milked. Young dairy cows in good body condition make good recipients and can be kept in the milking herd after delivering the calf.
Drost M (1984)

Beef Recipients.
A group of beef cattle recipients on pasture in South Florida. The cow in left front foreground appears in poor body condition and would not be a good prospect as a recipient. She is likely to be anestrous.
Drost M (1984)

Nymphomaniac Jersey Cow.
This Jersey cow is a nymphomaniac. She suffers from chronic cystic follicular degeneration and displays a sterility hump, an elevated tailhead, due to relaxation of the pelvic ligaments. Cows with chronic nymphomania do not make good donors, nor recipients.
Drost M (1969)

Nymphomaniac Friesian Cow.
This Friesian cow is a nymphomaniac. She suffers from chronic cystic follicular degeneration and displays a sterility hump, an elevated tailhead, due to relaxation of the pelvic ligaments. Cows with chronic nymphomania do not make good donors, nor recipients.
Utrecht (1976)
Grossly Obese Recipient.
This is an undesirable recipient. Overconditoned cows frequently have poor fertility. Epidural anesthesia is difficult to administer. Palpation of the reproductive tract is difficult due to perirectal fat.
Drost M (1986)

Morbidly Obese Angus Cow.
Infertile, morbidly obese Angus cow. These cows are very difficult to palpate because of the presence of excessive perirectal fat. Not suitable as a donor or a recipient.
Drost M (1986)

Limousin Donor Heifer.
Front cover of a Canadian Farm Journal in Dec 1972 proudly featuring a Limousin embryo donor heifer and her litter of calves produced in British Columbia. Both embryo recovery and embryo transfers were performed surgically.
Drost M (1972)

Holstein Donor with 8 Calves.
Holstein donor cow with her eight live calves.
Drost M (1984)

Transvaginal Transducer.
Transvaginal transducer with a sector scanner and a needle guide (7.5 MHz). This instrument may be used to ablate the dominant follicle at the beginning of the superovulatory treatment.
Drost M (1992)

Transvaginal Transducer - Close up.
Close up of a transvaginal transducer with a sector scanner and a needle guide (7.5 MHz). The needle is inserted into the dominant follicle to aspirate the follicular fluid and inactivate the dominant follicle.
Drost M (1992)

spiration Needle for Transvaginal Oocyte Recovery.
The needle guide and the movable tip of the aspiration needle are shown.
Drost M (1992)
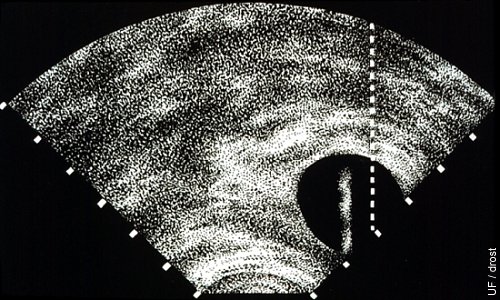
Ablation of the Dominant Follicle.
A vaginal transducer with sector scanner is used. The screen is equipped with a trajectory or target line for the aspiration needle. The follicle is lined up with the dotted line after which the aspiration needle is advanced until the tip becomes visible within the follicle.
Drost M (1992)
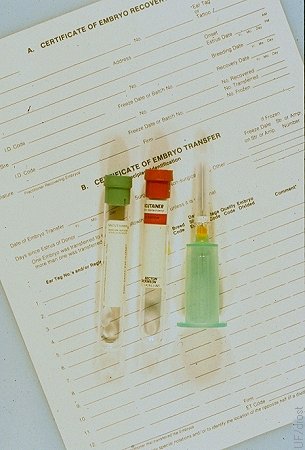
Blood Typing the Donor.
Donors should be blood typed for registration purposes and parentage determination of the embryo offspring. This should be done before or at the time of collection. Donors have been known to die before their calves were born.
Drost M (1980)
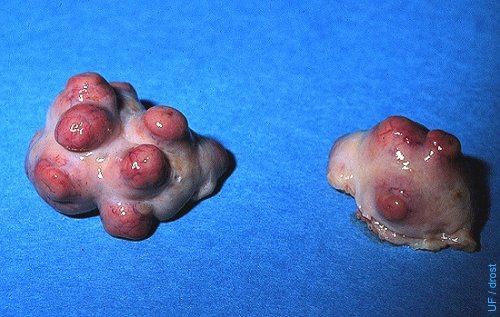
Superovulated Ovaries (FSH).
Nice superovulatory response: 7 corpora lutea on the left ovary and (only) 1 anovulatory follicle; 4 or 5 corpora lutea on the right ovary. Day 7, routine time for collection.
Drost M (1976)
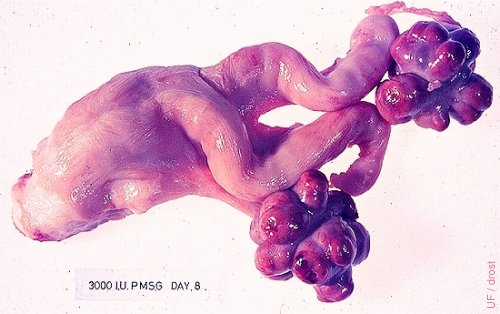
Superovulated Ovaries (eCG).
Excellent response to 3000 IU of eCG (formerly PMSG). The left ovary shows only one small anovulatory follicle. Heifer tract. eCG: equine Chorionic Gonadotropin PMSG: Pregnant Mare Serum Gonadotropin
Drost M (1976)
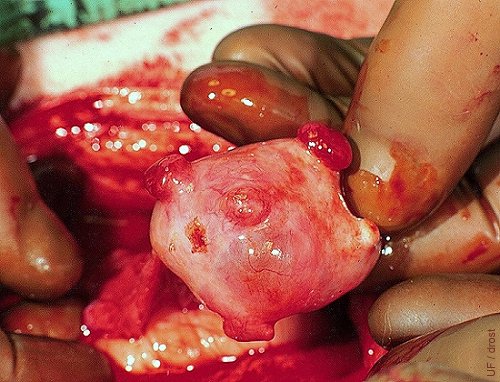
Superovulated Ovary on Day 3.
Three corpora hemorrhagica are readily visible. Also a corpus albicans at the lower tip of the thumb.
Drost M (1973)

Cervical Dilator.
Cervical dilator: diameter 6 mm, 50 cm long with a 4 cm tapered tip to a end diameter of 2.5 to 3.0 mm. Made out of stainless steel or aluminum alloy. Compared with a standard 45 cm long AI syringe.
Drost M (1984)
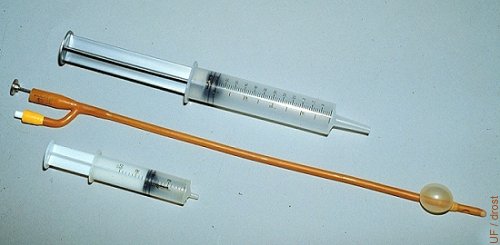
Foley Catheter.
Foley catheters are human urinary balloon catheters. They are 45 cm long and the diameter varies from 12 French gauge (1 French gauge = 0.3 mm) to 24 French gauge. A 20 French gauge is most commonly used in cows, while in heifer a 16 French gauge catheter is is used. The balloon is distended with air or with flushing medium; approximately 15 cc in cows, approximately 12 cc in heifers.
Drost M (1976
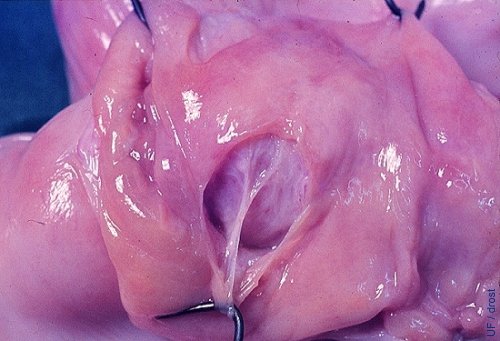
Endometrial Damage.
The endometrium can be split when the balloon of the Foley catheter is overdistended. When this occurs the flushing medium will infiltrate the wall of the uterus from where it cannot be recovered. Hemorrhage will further complicate recovery of the embryos.
Drost M (1975)
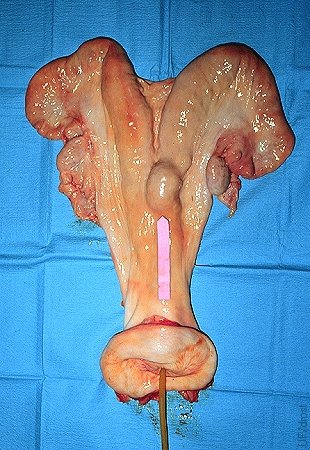
Location of the Balloon.
The local distension at the base of the right uterine horn indicates the location of the inflated balloon.
Drost M (1975)
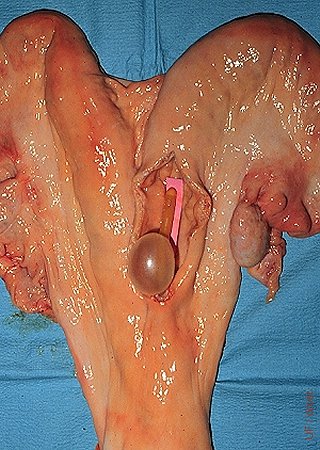
Balloon Placement.
The balloon is placed at the base of the horn for a unilateral flush.
Drost M (1975)
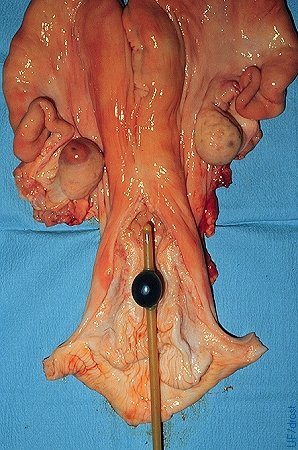
Bilateral Uterine Flush.
The balloon may be placed in the anterior portion of the cervix with the tip of the catheter in the uterine body to flush both horns simultaneously. Inflation of the balloon in the cervix meets with more resistance, compared with placement in the uterus.
Drost M (1975)

Nonsurgical Uterine Flush.
Day 7, nonsurgical flush by gravity flow. The free hand of the palpator controls the inflow and outflow. The effluent is passed through an embryo filter / concentrator. One liter of flushing medium is routinely used. The level of fluid in the embryo filter is controlled with the blue clamp.
Drost M (1995)

Elevation of the Chute.
The chutes have been slightly elevated in the rear to allow the viscera to fall forward and be more readily accessible during surgical transfer.
Drost M (1975)
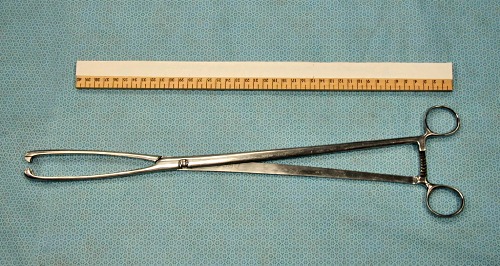
Cervical Forceps.
Cervical forceps (45 cm long)for the retraction of the cervix of a pendulous uterus, to facilitate placement of the flushing catheter.
Drost M (2009)
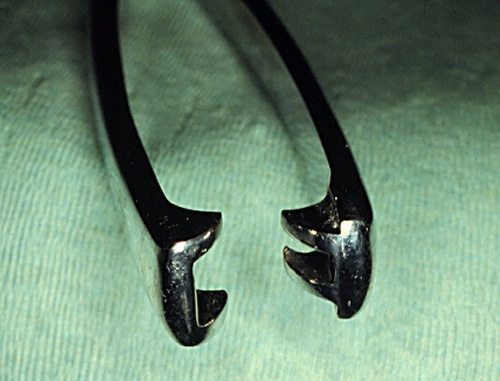
Jaws of Cervical Forceps.
Relatively atraumatic jaws of a long cervical forceps.
Drost M (1975)
Cervical Forceps Applied.
Only only one jaw of the forceps is inserted into the cervix taking a deep, firm bite to avoid tearing off a small piece of tissue during retraction.
Drost M (1975)
Cervical Forceps and Catheter in Place.
One of the jaws of the cervical forceps is inserted directly into the cervical canal. After retraction of the cervix, the Foley catheter is introduced alongside this jaw.
Drost M (1975)

Graduated Cylinder.
A 500 ml or 1000 ml sterile graduated cylinder may be used to collect the flushing medium. The embryos will settle to the bottom by gravity. After a 15 minute wait the upper portion of the fluid may be gently siphoned off by lowering a small diameter length of tubing to the 50 mm mark. The remaining smaller volume may then be poured into a searching dish and examined under the microscope for the presence of embryos. This method is less expensive than the use of single-use disposable embryo filters.
Drost M (1980)

Embryo Filter.
Embryo filter (EmCon) with a stainless steel sieve. Diameter of the pores is 75 micrometers. The white clamp on the drainage tube controls the level of flushing medium in the filter. The effluent tube of the flushing catheter can be connected directly to the nipple on the lid of the filter.
Drost M (1980)
Rinsing the Filter.
The filter is rinsed with plain flushing medium (no serum) to keep it from foaming. A 25 ga needle is used and the contents of the filter are poured into a searching dish with a grid, and marked with the number of the donor.
Drost M (1980)
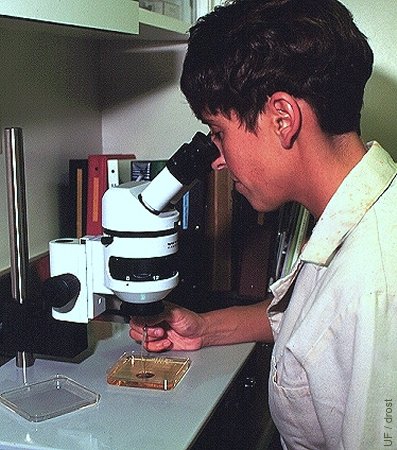
Searching for Embryos.
Searching for embryos under the stereoscope at a magnification of 40 to 70 X.
Drost M (1995)
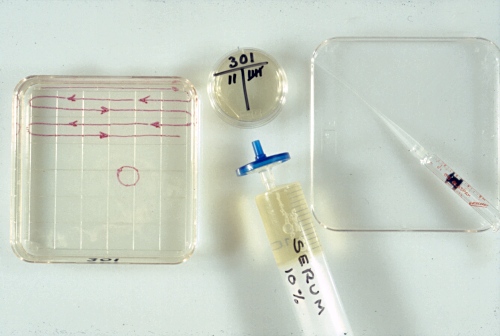
Searching Dish.
The square, sterile, searching dish is filled half full of flushing medium which contains 1% fetal calf serum. The holding medium in the round holding dish contains 10% fetal calf serum.
Drost M (1980)
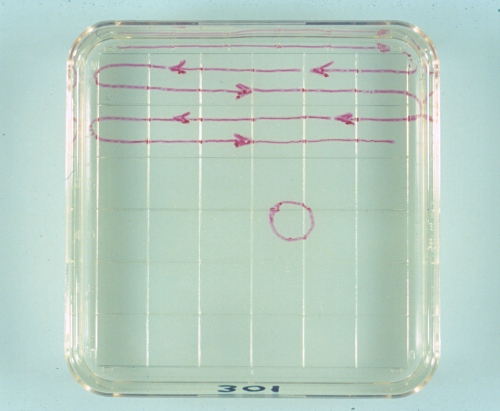
Searching Pattern.
The circle indicates the size of the viewing field at 70 X magnification. A systematic search is conducted along the top half of the 1cm squares from left to right, and then along the bottom half from right to left. The number of the donor is written on the bottom part of the searching dish.
Drost M (1980)
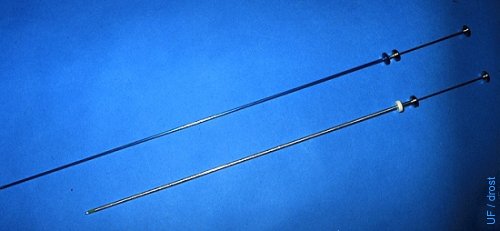
Embryo Transfer Syringe.
Comparison of a 21 inch (53 cm) embryo transfer syringe with an 18 inch (45 cm) artificial insemination syringe.
Drost M (1980)

0.25 cc AI Syringe.
0.25 cc AI syringe with O ring and split end sheath. A 0.25 cc straw loaded with an embryo in the center column of medium between to air pockets is also shown.
Drost M (1974)
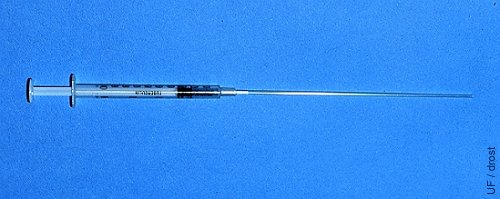
TB Syringe with Straw.
The cotton plug end of a sterile 0.25 cc straw is inserted directly into the tip of a 1.0 ml tuberculin syringe.
Drost M (1980)
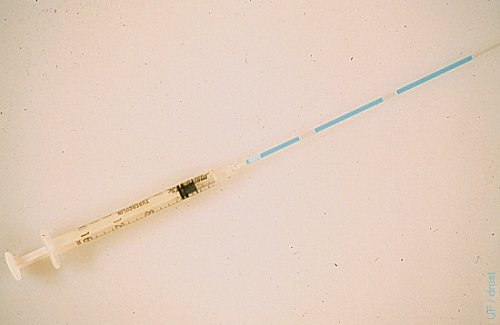
Straw Loaded with Embryo.
A 0.25 cc sterile straw is attached to a 1 ml tuberculin syringe by inserting the plug end directly into the tip of the syringe. After rinsing the straw with sterile transfer medium the straw is sequentially loaded with a 2 cm column of medium, a 1 cm column of air, a 3 cm column of medium containing the embryo, 1 cm column of air, and 2 cc column of medium.
Drost M (1980)

ET Syringe with Sanitary Sleeve.
Comparison of a 53 cm embryo transfer syringe with a standard 45 cm artificial insemination syringe. The transfer syringe is covered with a sanitary sleeve.
Drost M (1980)
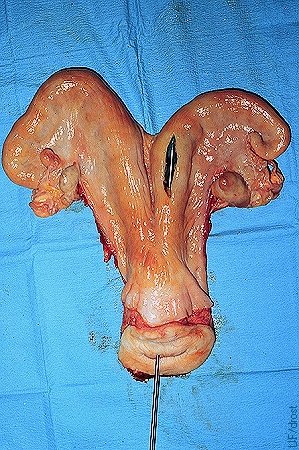
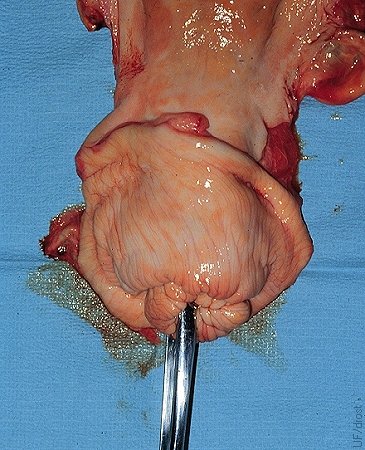
Nonsurgical Embryo Placement.
Preferred location for the nonsurgical placement of the embryo in the horn ipsilateral to the corpus luteum, at the level of the external bifurcation of the horn, about one-third of the distance up the horn. The tip of the transfer instrument can be seen through the incision in this slaughterhouse specimen. Deeper placement may be ideal, but excessive manipulation and trauma to the endometrium must be avoided.
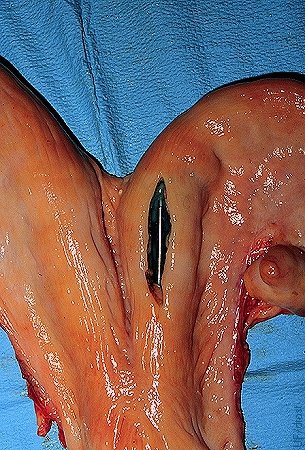
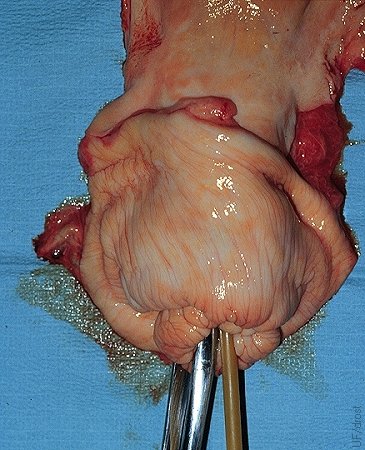
Nonsurgical Embryo Placement.
Close-up of the preferred location for the nonsurgical placement of the embryo in the horn ipsilateral to the corpus luteum, at the level of the external bifurcation of the horn.
Drost M (1980)
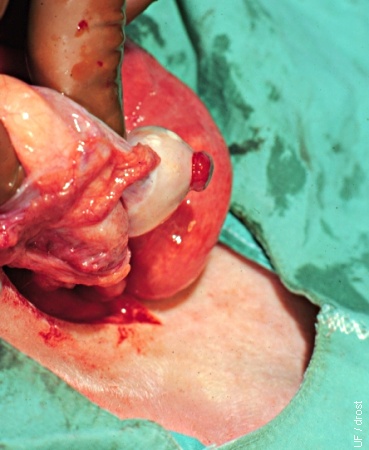
Surgical Transfer on Day 3.
The Day 3 corpus hemorrhagicum is directly, visually evaluated during surgical transfer.
Drost M (1974)
Surgical Embryo Placement.
Preferred location for the surgical placement of the embryo is in the distal tip of the horn ipsilateral to the corpus luteum, 5 to 10 cm from the tubo-uterine junction.
Drost M (1974)

Transfer via the Flank.
Surgical transfer via the paralumbar fossa to the tip of the right horn ipsilateral to the ovary bearing the corpus luteum, which had been identified by transrectal palpation prior to the surgery.
Drost M (1974)
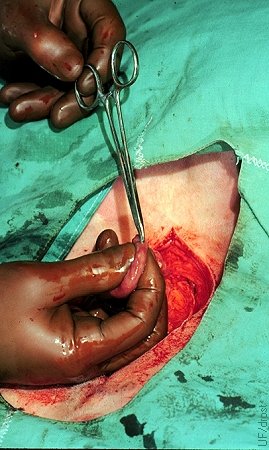
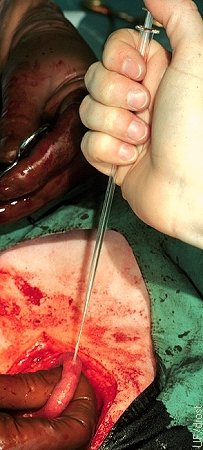
Site of Surgical Transfer.
A stab incision is made with a mosquito forceps along the greater curvature of the uterine horn. Uterine blood supply is minimal at this location. The embryo is subsequently introduced with a Tomcat catheter and deposited in the lumen 2 to 3 cm away from the stab incision to avoid contact with blood or serum.
Drost M (1974)
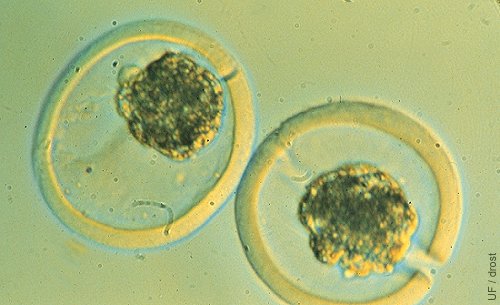
Embryo Splitting.
Two semi-embryos after splitting of a morula. One semi-embryo was placed in an empty surrogate zona pellucida. Notice the slits in the zonae pellucidae.
Seidel GE (1984)

Identical Twins.
Two sets of identical twins produced by embryos splitting at Colorado State University.
Seidel GE (1984)