The Visual Guide to
Bubaline Reproduction
Reproductive Technology: Embryo Transfer

Overview of Embryo Recovery.
The donor is confined in the stocks. The Foley catheter, placed under epidural anesthesia, is connected to the flushing medium suspended approximately 1 meter above the level of the uterus. The return-flow tubing is directed to an embryo filter.
Drost M (1984)

Ready to Place Catheter.
The donor has received epidural anesthesia and is now ready for the placement of the sterile catheter.
Drost M (1984)

Cervical Dilator.
Cervical dilator: diameter 6 mm, 50 cm long with a 4 cm tapered tip to an end diameter of 2.5 to 3.0 mm. Made out of stainless steel or aluminum alloy. Compared with a standard 45 cm AI syringe.
Drost M (1984)
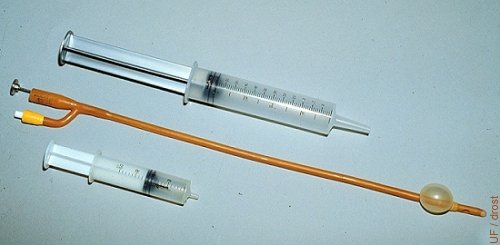
Foley Catheter.
Foley catheters are human urinary balloon catheters. They are 45 cm long and the diameter varies from 12 French (1 French = 0.3 mm) to 24 French. A 20 French size is most commonly used in cows, while in heifers a 16 French is used. The balloon is distended with air or with flushing medium; ~15 cc in cows, ~12 cc in heifers.
Drost M (1984)
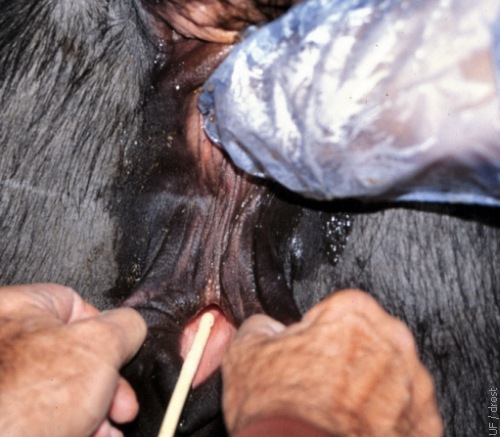
Insertion of the Foley Catheter.
An assistant parts the lips of the vulva by pulling them posteriorly and laterally, thereby creating a tunnel and minimizing the vulvovaginal sphincter. The Foley catheter is now ready to be advanced.
Drost M (1984)
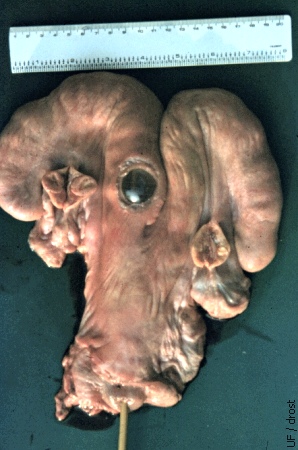
Balloon Placement.
A window is cut in the wall of this slaughterhouse specimen to illustrate the location of the inflated balloon at the base of the horn.
Vale WG (1987)
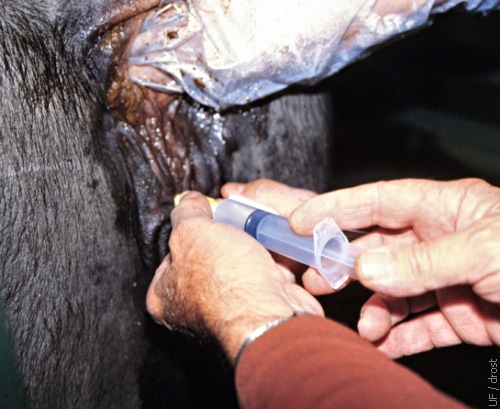
Inflation of the Balloon.
The balloon of the Foley catheter is inflated with 12 to 15 cc of air (or distension with sterile flushing medium).
Drost M (1984)
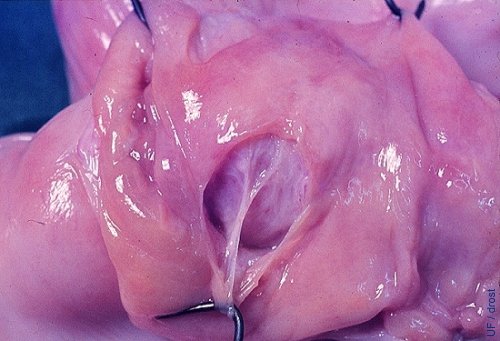
Endometrial Damage.
The endometrium can be split when the balloon of the Foley catheter is overdistended. When this occurs the flushing medium will infiltrate the wall of the uterus from where it cannot be recovered. Hemorrhage will further complicate recovery of the embryos (bovine demonstration specimen).
Drost M (1984)
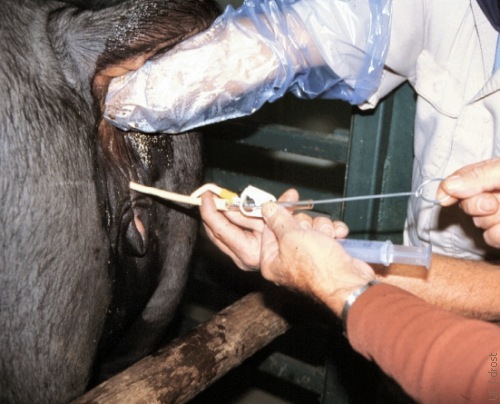
Removal of the Stylet.
After the Foley catheter has been placed and the balloon has been inflated, the stylet is removed.
Drost M (1984)
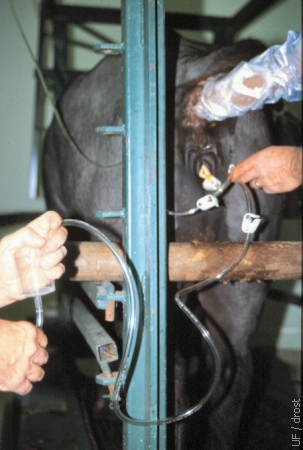
Exit Tubing.
The in-flow and the out-flow can be regulated with squeeze-type clamps on the tubing. During out-flow the filter should be held at a level below the uterus to facilitate gravity flow.
Drost M (1984)

Return Flow to the Filter.
The effluent is directed to the embryo filter which retains the embryos but allows the excess fluid and occasional blood to flow through.
Drost M (1984)

Embryo Filter.
Embryo filter (EmCon) with a stainless steel sieve. Diameter of the pores is 75 micrometers. The white clamp on the drainage tube controls the level of flushing medium in the filter. The effluent tube of the flushing catheter is connected directly to the nipple on the lid of the filter.
Drost M (1984)

Flow Control Through the Filter.
The amount of fluid in the filter can be controlled with squeeze clamp below the filter. A layer of 1 to 2 ml of fluid should always be kept in the filter to prevent the embryos from becoming dehydrated.
Drost M (1984)
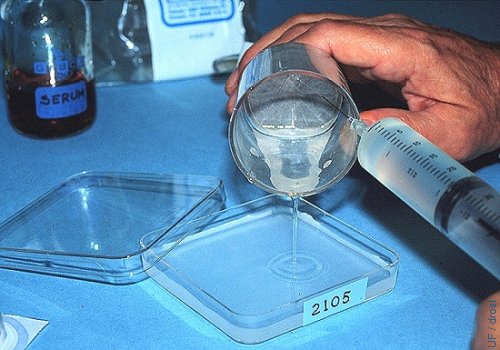
Rinsing the Filter.
The filter is rinsed with plain flushing medium (no serum) to keep it from foaming. A 25 ga needle is used and the contents of the filter are poured into a searching dish with a grid and marked with the number of the donor.
Drost M (1984)

Embryo Collection.
With the superovulated donor in the chute embryos are collected nonsurgically by gravity flow into a graduated cylinder. Routinely 1 liter of phosphate buffered saline is used per flush.
Drost M (1984)

Graduated Cylinder.
A 500 ml or 1000 ml sterile graduated cylinder may be used to collect the flushing medium. The embryos will settle to the bottom by gravity. After a 15 minute wait the upper portion of the fluid may be gently siphoned off by lowering a small diameter length of tubing to the 50 mm mark. The remaining smaller volume may then be poured into a searching dish and examined under the microscope for the presence of embryos. This method is less expensive than the use of single-use disposable embryo filters.
Drost M (1984)
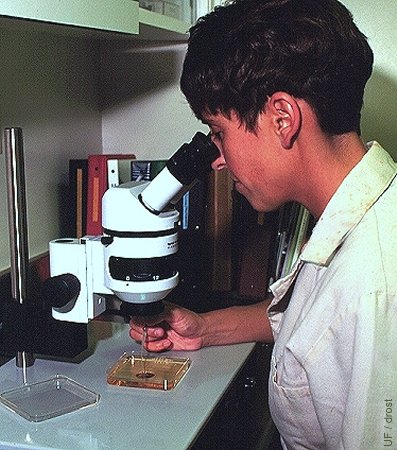
Searching for Embryos.
Searching for embryos under the stereoscope at a magnification of 40 to 70 X.
Drost M (1984)
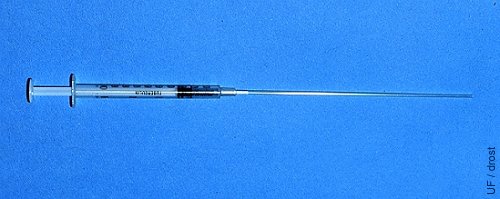
TB Syringe with Straw.
The cotton plug end of a sterile 0.25 cc straw is inserted directly into the tip of a 1.0 ml tuberculin syringe.
Drost M (1984)
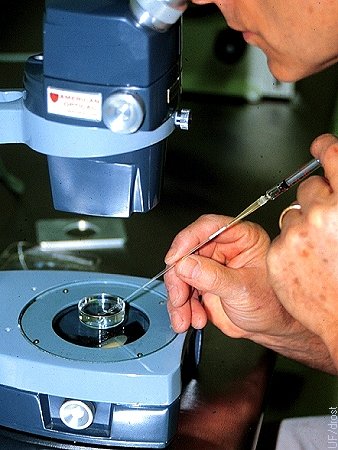
Loading the Straw.
Aspirating the embryo from the holding dish into a 0.25 cc straw attached to a tuberculin syringe.
Drost M (1984)
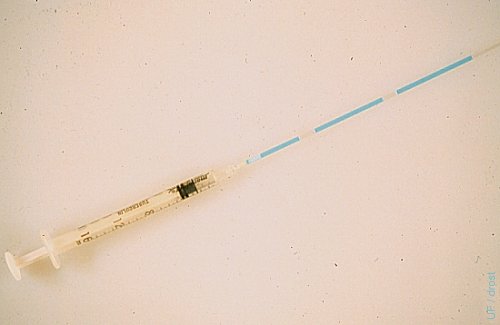
Straw Loaded with Embryo.
A 0.25 cc sterile straw is attached to a 1 ml tuberculin syringe by inserting the plug end directly into the tip of the syringe. After rinsing the straw with sterile transfer medium the straw is sequentially loaded with a 2 cm column of medium, a 1 cm column of air, a 3 cm column of medium containing the embryo, 1 cm column of air, and 2 cc column of medium.
Drost M (1984)
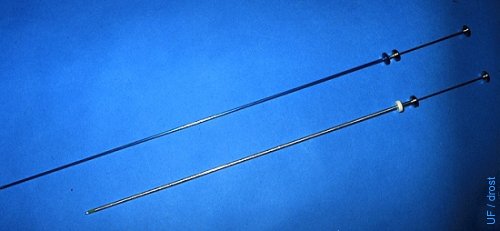
Embryo Transfer Syringe.
Comparison of a 21-inch (53 cm) embryo transfer syringe with a standard 18-inch (45 cm) artificial insemination syringe.
Drost M (1984)

Standard AI Syringe.
0.25 cc AI syringe with O ring and split end sheath. A 0.25 cc straw loaded with an embryo in the center column of medium between two air pockets is also shown.
Drost M (1984)

ET Syringe with Sanitary Sleeve.
Comparison of a 53 cm embryo transfer syringe with a standard 45 cm artificial insemination syringe. The transfer syringe is covered with a sanitary sleeve.
Drost M (1984)
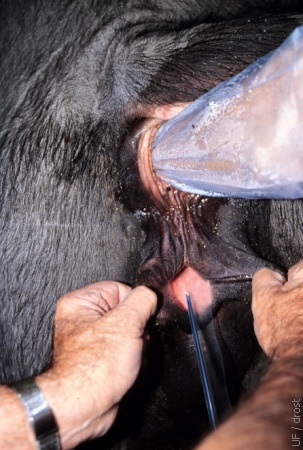
Parting of the Lips of the Vulva.
An assistant parts the lips of the vulva by pulling them posteriorly and laterally, thereby creating a tunnel and minimizing the vulvovaginal sphincter. The transfer syringe can now be inserted into the vagina.
Drost M (1984)
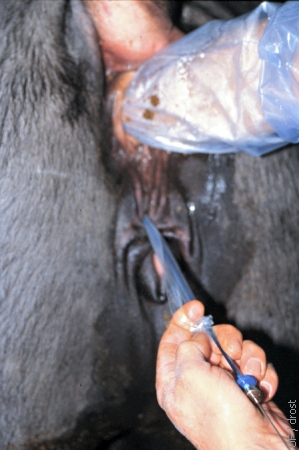
Perforating the Sanitary Sleeve.
Just before introducing the transfer syringe into the external cervical os, the sanitary sleeve is pulled back over the tip of the syringe.
Drost M (1984)
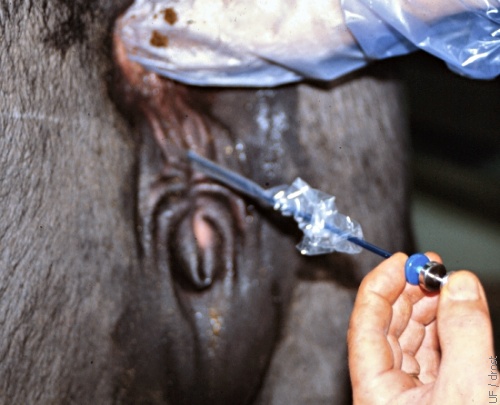
Deposition of the Embryo.
The embryo is placed in the uterine horn ipsilateral to the corpus luteum, at the level of the external bifurcation, approximately one-third of the way into the uterine horn.
Drost M (1984)
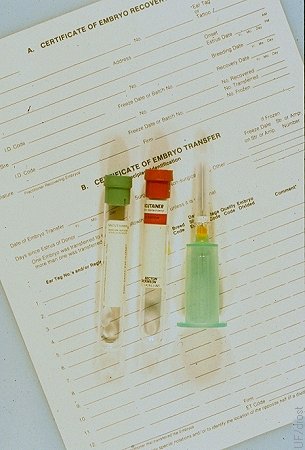
Blood Typing the Donor.
Donors should be blood typed for registration purposes and parentage determination of the embryo offspring. This should be done before or at the time of collection. Donors have been known to die before the recipient gave birth to her calf.
Drost M (1984)

World's First ET Calf.
The first buffalo calf produced by embryo transfer was born at the University of Florida on 18 March 1983. The donor was a Swamp buffalo, the recipient a Jafarabadi buffalo.
Drost M (1983)

World's First ET Calf.
The world's first embryo transfer calf held by Dr. Wyland S. Cripe, veterinarian at the University of Florida. Born on 18 March 1983.
Drost M (1983)

First ET Calf.
The first buffalo calf produced by embryo transfer was born at the University of Florida on 18 March 1983. The donor was a Swamp buffalo, the recipient a Jafarabadi buffalo.
Drost M (1983)

The First ET Calves in Bulgaria.
Three of the first group of buffalo embryo transfer calves in Bulgaria, and in Europe, born in 1986.
Drost M (1986)

ET Working Area Setup.
Work area next to the hydraulic holding chute for the donor animal. It is complete with an ultrasound unit and a microscope for searching the collected effluent.
Zambrano-Varon J (2012)
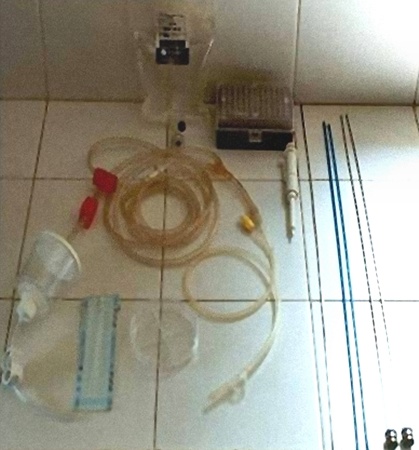
ET Supplies.
Embryo filter attached to the flushing medium and the collecting Foley catheter with a searching dish. To the right: two transfer syringes and two blue sheaths.
Zambrano-Varon J (2012)

Foley Catheter Tip.
The tip of the Foley catheter is rounded and closed at the end. There are two lateral openings 1 to 2 cm from the tip. Proximal to the openings is an uninflated balloon.
Zambrano-Varon J (2012)
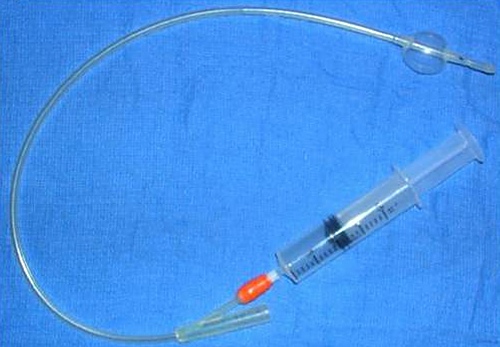
Inflated Balloon.
The balloon proximal to the tip of the Foley catheter has been inflated via the orange port.
Zambrano-Varon J (2012)
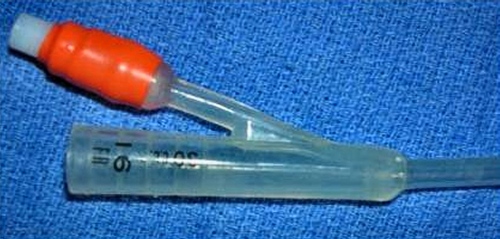
Proximal End of Catheter.
The balloon proximal of the Foley catheter. The orange port is to inflate the balloon via its designated thin channel. The proximal end of the catheter accommodates the end of a syringe or a flushing medium tube with an adapter.
Zambrano-Varon J (2012)

Embryo Filter.
The embryo filter has a sieve [mesh] with openings of 75 micrometers which permit fine debris, including erythrocytes (diameter 7-8 micrometers), to flow through but retains the embryos and unfertilized ova. The level of flushing medium in the filter should be maintained at no less than approximately 1 cm from the bottom to prevent the embryos from drying out on an empty screen.
Zambrano-Varon J (2012)

Rinsing the Filter.
The embryo filter is gently rinsed with the aid of a syringe and needle while the flushing medium is poured into a searching dish.
Zambrano-Varon J (2012)

Pouring Out the Filter.
The flushing medium is gently poured into a searching dish.
Zambrano-Varon J (2012)

Filter Mesh.
The diameter of the pores in the mesh of the filter is 75 micrometers which retains the embryos and oocytes (diameter 110 to 120 micrometers) but allows erythrocytes (diameter 7 to 8 micrometers) to flow through.
Zambrano-Varon J (2012)

Nonsurgical Transfer.
Actual nonsurgical, transcervical transfer of the embryo to a synchronized recipient on Day 6.
Zambrano-Varon J (2012)
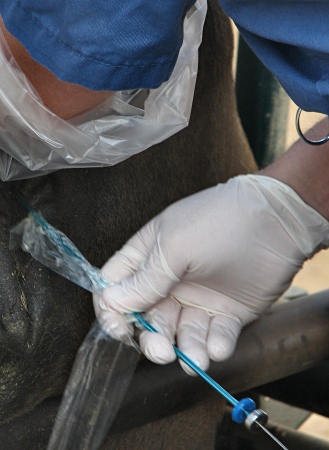
Sanitary Sleeve.
The embryo transfer syringe is inserted in a closed sterile sanitary sleeve prior to its introduction into the vagina to prevent contamination of its tip prior to entering the external os of the cervix.
Zambrano-Varon J (2012)
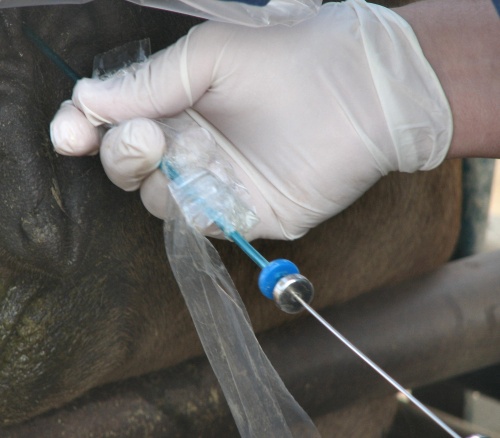
Sterile Insertion.
Once the tip of the transfer syringe, covered by the sanitary sleeve, has entered the cervix the sanitary sleeve is pulled back and the ET rod is advanced to puncture the sleeve.
Zambrano-Varon J (2012)
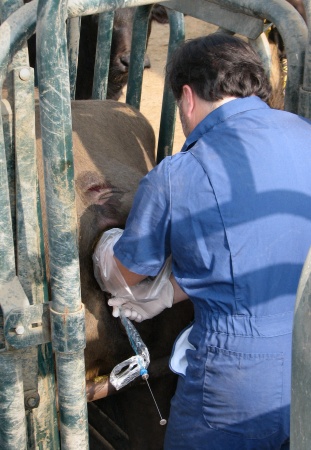
Transcervical Transfer.
Nonsurgical transfer of the embryo to a synchronized recipient on Day 6.
Zambrano-Varon J (2012)
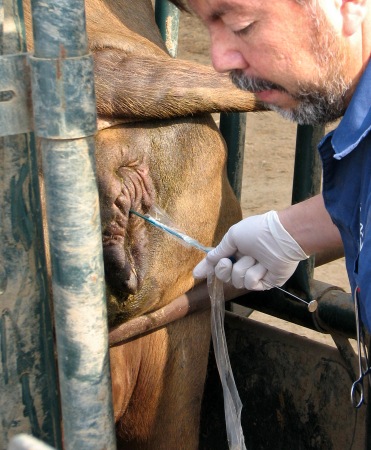
Retraction of the Sleeve.
The sanitary sleeve has been retracted while advancing the transfer rod through the cervix.
Zambrano-Varon J (2012)

Embryo Recovery.
Nonsurgical recovery of embryos. The bag with the flushing medium is suspended about 1 meter above the level of the uterus to enable gravity flow. The embryo filter is held below the level of the uterus, usually by an assistant.
Zambrano-Varon J (2012)

Connection of the Tubing.
The flushing medium enters the lumen of the uterus via a Foley catheter which is connected to one arm of a Y connector while the outflow tube, connected to the other arm of the connector, is clamped off. Once the uterus has been distended the inflow is clamped off and the clamp on the outflow tube is released. Shown is the black clamp on outflow tube
Zambrano-Varon J (2012)

Foster Cows.
These Holstein cows serve as foster cows to nurse the buffalo ET calves. The Holstein cows were not the recipients of the buffalo embryos. Transfer of embryos from one genus [Bubalus bubalis] to another genus [Bos taurus] is not possible.
Zambrano-Varon J (2012)
